PDF chapter test TRY NOW
The term 'element' was first used bya scientist named Robert Boyle. He is a supporter of the elemental essence of matter and the nature of vacuum from the beginning. Boyle's Law was his best-known work.

Robert Boyle
Example:
The simplest form of matter is an element. In our everyday lives, we use a variety of components.
- Water is made up of two elements: Hydrogen and oxygen. Magnesium and Phosphorus are used in the production of crackers. In agriculture, sulphur is used as a fertiliser.
- The elements sodium and chlorine are found in common salt.
- Mobile phones are made of gallium, while computer chips are made of silicon.
Important!
Note: To date, \(118\) elements have been identified. \(94\) of these elements are found in nature, while \(24\) have been produced artificially in the lab.
Elements are the fundamental substances that cannot be separated into simpler substances by any chemical methods. All the elements in the periodic table is an example for elements such as oxygen, iron, etc.
According to Antoine Laurent Lavoisier, an element is a fundamental type of matter that cannot be broken down into simpler substances by chemical reactions.
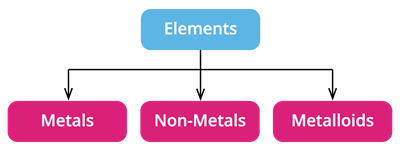
Based on their chemical properties, we can divide the elements into metals, non-metals, and metalloids.
- Metalloids: They have properties that are similar to both metals and non-metals.
- There are around \(8\) elements in the periodic table that are called as Metalloids.
Example:
Metalloids include arsenic, antimony, silicon, and boron, to name a few.
Let's discuss metals, and non-metals in the upcoming sections.
