
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoHow do we find the acid and base used in the formation of the given salts?
With the help of positive and negative radicles, we can find the acid and base that is reacted to form the respective salts, as the positive radical is dissociated from the base and the negative radical from the acid.
With the help of positive and negative radicles, we can find the acid and base that is reacted to form the respective salts, as the positive radical is dissociated from the base and the negative radical from the acid.
Example:
Let us test the solubility of salt samples given with the help of pH paper:
Salt samples:
- Sodium chloride
- Potassium nitrate
- Aluminium chloride
- Zinc sulphate
- Copper sulphate
- Sodium acetate
- Sodium carbonate
- Sodium hydrogen carbonate
- Ammonium chloride
Salt | pH | Base | Acid | Solubility |
Sodium chloride | \(7\) | \(NaOH\) | \(HCl\) | Completely soluble |
Potassium nitrate | \(7\) | \(KOH\) | \(HNO_3\) | Completely soluble |
Aluminium chloride | \(<7\) | \(Al(OH)_3\) | \(HCl\) | Completely soluble |
Zinc sulphate | \(<7\) | \(Zn(OH)_2\) | \(H_2SO_4\) | Completely soluble |
Ammonium chloride | \(<7\) | \(NH_4OH\) | \(HCl\) | Completely soluble |
Copper sulphate | \(<7\) | \(Cu(OH)_2\) | \(H_2SO_4\) | Completely soluble |
Sodium acetate | \(>7\) | \(NaOH\) | \(H_2CO_3\) | Completely soluble |
Sodium carbonate | \(>7\) | \(NaOH\) | \(H_2CO_3\) | Completely soluble |
Sodium hydrogen carbonate | \(>7\) | \(NaOH\) | \(H_2CO_3\) | Completely soluble |
From the above table, we can say that all the given salt samples are completely soluble, and the reactants used for the preparation of these samples and their pH are recorded.
Note: The pH may vary according to the concentration taken, and the above-given pH is the general range of those salts.
The nature of the salt can be determined by the reactants used for the preparation of these salts i.e.
- \(\text{Strong acid + Strong base → Neutral Salt + Water}\)
- \(\text{Weak acid + Weak base → Neutral Salt + Water}\)
- \(\text{Strong acid + Weak base → Acidic Salt + Water}\)
- \(\text{Weak acid + Strong base → Basic Salt + Water}\)
Chemicals from common salt \(NaCl\):
We know the process of salt formation and its use in our homes.
\(NaOH + HCl \rightarrow NaCl + H_2O\)
Seawater is a mixture of many salts. These salts are separated to produce sodium chloride. Solid salt deposits can also be found in various parts of the world. These large crystals are frequently brown because of impurities. This is known as rock salt. Rock salt beds were formed when ancient seas dried up. These rock salt is mined in the same way as coal.
Note: Mahatma Gandhi’s Dandi March: Sodium chloride played a prominent part in our fight for freedom.
\(NaCl\) - A raw material for chemicals:
Common salt is an important raw material for many substances that we use in our daily life, including sodium hydroxide, baking soda, washing soda, bleaching powder, and many others.
Let us take a look at how \(NaCl\) is used to create all of these different substances:
1. Sodium hydroxide \(NaOH\):
The Chlor-alkali process is an industrial process that produces chlorine and sodium hydroxide by electrolysis of sodium chloride solution.
The anode emits chlorine gas, while the cathode emits hydrogen gas. Near the cathode, a sodium hydroxide solution is formed.
The overall reaction that occurs during the Chlor-alkali process is as follows:
The overall reaction that occurs during the Chlor-alkali process is as follows:
\(2NaCl + H_2O \rightarrow 2NaOH + Cl_2 + H_2\)
The three by-products of this process are all useful. For example,
\(H_2\) - Used as fuels, margarine, ammonia for fertilisers
\(Cl_2\) - Used for water treatment, swimming pools, PVC, disinfectants, CFCs and pesticides etc.
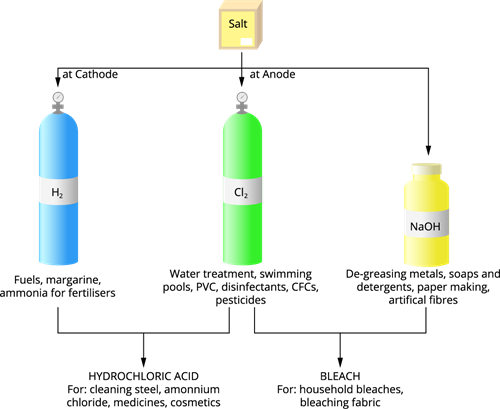
Important products from the Chlor-alkali process
Properties and uses of \(NaOH\):
- It is insoluble in non-polar solvents.
- \(NaOH\) is deliquescent and readily absorbs \(CO_2\) from the air.
- Used in petroleum refining.
- Used as drain cleaner.
- Used in the preparation of sodium metal and many salts of the metals.
- Used in de-greasing metals, soaps, detergents, paper making and artificial fibres.
2. Bleaching powder:
In \(1799\), the Scottish chemist Charles Tennant invented bleaching powder, a solid mixture of chlorine and slaked lime.
When chlorine is passed over solid slaked lime for an extended period of time, bleaching powder is formed. It is also known as lime chloride.
The chemical formula for bleaching powder is \(CaOCl_2\), but the actual composition is quite complex.
\(Ca(OH)_2 + Cl_2 \rightarrow CaOCl_2 + Cl_2 + H_2O\)
Note: In the laboratory, bleaching powder is prepared by pouring a freshly prepared slaked lime into a gas jar full of chlorine and shaking this mixture. The chlorine's colour vanishes almost instantly. Before absorption is complete, the product can be used to absorb chlorine from several more gas jars.
Properties and uses of \(CaOCl_2\):
- It is a yellow-white solid with a strong smell of chlorine.
- Used as a bleaching agent.
- Used as a disinfectant for drinking water and also as a sanitiser for swimming pools.
- Used in the manufacturing of chloroform.
Note: Bleaching powder dissolves in water forming hypochlorous acid, which has effective germicidal property
\(Ca(OCl)_2 + 2H_2O \rightarrow 2HOCl_2 + Ca(OH)_2\)
3. Sodium hydrogen carbonate or Baking soda \(NaHCO_3\):
Baking soda is a common ingredient in the preparation of tasty, crispy pakoras. It is sometimes added to speed up the cooking process. The compound's chemical name is sodium hydrogen carbonate \(NaHCO_3\). One of the raw materials used in its production is sodium chloride.
\(NaCl + H_2O + CO_2 + NH_3 \rightarrow NH_4Cl + NaHCO_3\)
Sodium bicarbonate is produced as an intermediate product in Solvay's process for producing sodium carbonate, where the soluble sodium bicarbonate crystallises.
\(Na_2CO_3 + H_2O + CO_2 \rightarrow 2NaHCO_3\)
Properties and uses of \(NaHCO_3\):
- It is a white crystalline powder.
- It is weakly alkaline in nature.
- Baking powder is made from baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. The following reaction occurs when baking powder is heated or mixed with water.
\(NaHCO_3 + H^+ \rightarrow CO_2 + H_2O + \) Sodium salt of acid
The carbon dioxide produced during the reaction causes bread or cake to rise, resulting in soft and spongy bread or cake.
- Used in deodorants for its neutralising action.
- It is used in fire extinguishers as an abrasive cleaner.
- Antacids contain sodium hydrogen carbonate because it is alkaline. It neutralises excess stomach acid and provides relief.
4. Sodium carbonate or Washing soda \(Na_2CO_3 . 10H_2O\):
Sodium carbonate exists in various forms, such as
- Anhydrous sodium carbonate, also known as soda ash \(Na_2CO_3\)
- Monohydrate sodium carbonate or crystal carbonate \(Na_2CO_3 . H_2O\)
- Heptahydrate sodium carbonate \(Na_2CO_3 . 7H_2O\)
- Decahydrate sodium carbonate or washing soda or sal soda \(Na_2CO_3 . 10H_2O\)
Methods of preparation:
Nowadays, sodium carbonate is typically produced using the ammonia soda or Solvay process. The ingredients for this process are readily available and reasonably priced.
These are sea salt brine (NaCl), ammonia (NH3), and limestone CaCO3 (from mines). The process is divided into several sections, and CaCl2 is a significant by-product.
Washing soda can also be produced by the recrystallization of sodium carbonate.
\(Na_2CO_3 + 10H_2O \rightarrow Na_2CO_3 . 10 H_2O\)
Properties and uses of \(Na_2CO_3 . 10 H_2O\):
- It is a white crystalline solid.
- It is readily soluble in water.
- Used in manufacturing of glass, borax, soap, caustic soda and so on.
- Used in paint, paper and textile industries.
- Used for softening of hard water.
- In the home, it is used as a cleaning agent.