
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAcid-base balance is pivotal for our health and the environment. We unknowingly perform many neutralisation processes in our daily lives. Let us now explore the significance of some of those reactions.
Do you know why baking soda is applied for insects bite?
Applying the baking soda neutralises and reduce the effect of the ant's sting; because the ant's sting contains formic acid, baking soda (sodium hydrogen carbonate) is a basic substance capable of neutralising the wound.
Applying the baking soda neutralises and reduce the effect of the ant's sting; because the ant's sting contains formic acid, baking soda (sodium hydrogen carbonate) is a basic substance capable of neutralising the wound.

Effect of formic acid
Bee bite:
Bees and red ants both contain formic acid. When bees or red ants bite humans, they send acid into our bodies. This acid creates pain and a burning sensation. A suitable base in the form of calcium hydroxide (lime paste available at home) is applied to neutralise the formic acid and reduce the pain.
Wasp bite:
When a wasp bites us, it injects an alkaline substance into our bodies, causing pain and a burning sensation. To neutralise the alkalinity, we add vinegar, which is an acid.
Note: The stinging hair of nettle leaves injects methanoic acid into the body, causing burning pain.
Nettle is a herbaceous plant that grows wild. Its leaves have stinging hairs that cause painful stings when accidentally touched. This is because they secrete methanoic acid. Rubbing the affected area with the leaf of the dock plant, which grows alongside the nettle in the wild, is a traditional remedy for it.
Tooth decay:
Doctors generally recommend that we wash our teeth twice a day. This is because the bacteria in our mouth decompose the food particles trapped between our teeth, resulting in the development of acid, which causes tooth decay.
Tooth decay
To prevent this, we have to neutralise the acid. So when we brush with tooth powder or toothpaste containing weak bases, the acid gets neutralised. As a result, we will have healthy and strong teeth. Most of the substances we use are basic, which do not harm the skin.
Note: When the pH of the mouth falls below \(5.5\), tooth decay progresses.
Acidity:
As we all know, the hydrochloric acid in our stomach helps in the digestion of food. Due to the excessive production of hydrochloric acid in our stomach, we feel a burning sensation in the food pipe and the chest area. If it prolongs for a long time, the ulcer will be formed in the stomach and food pipe, which further aggravates the conditions.
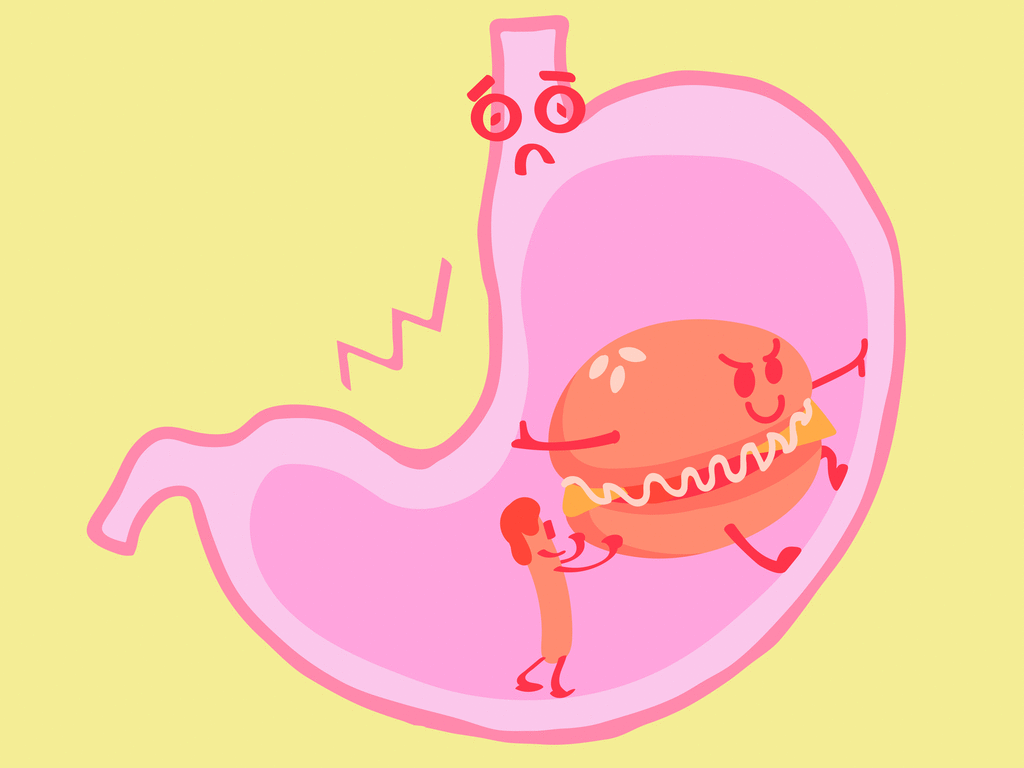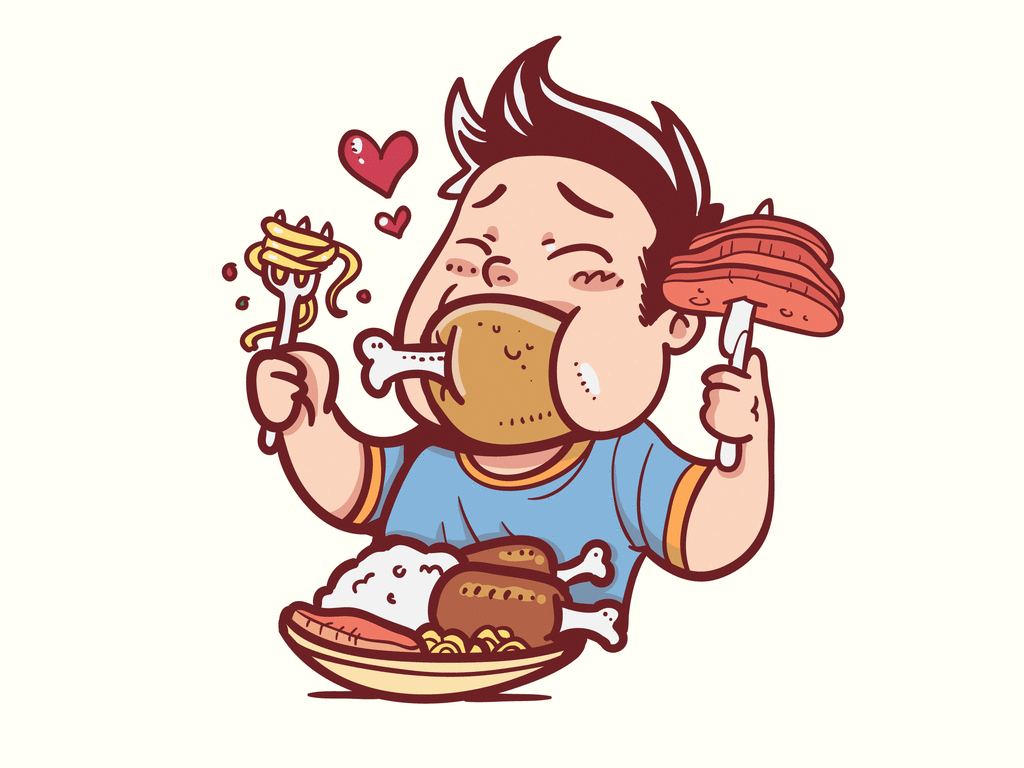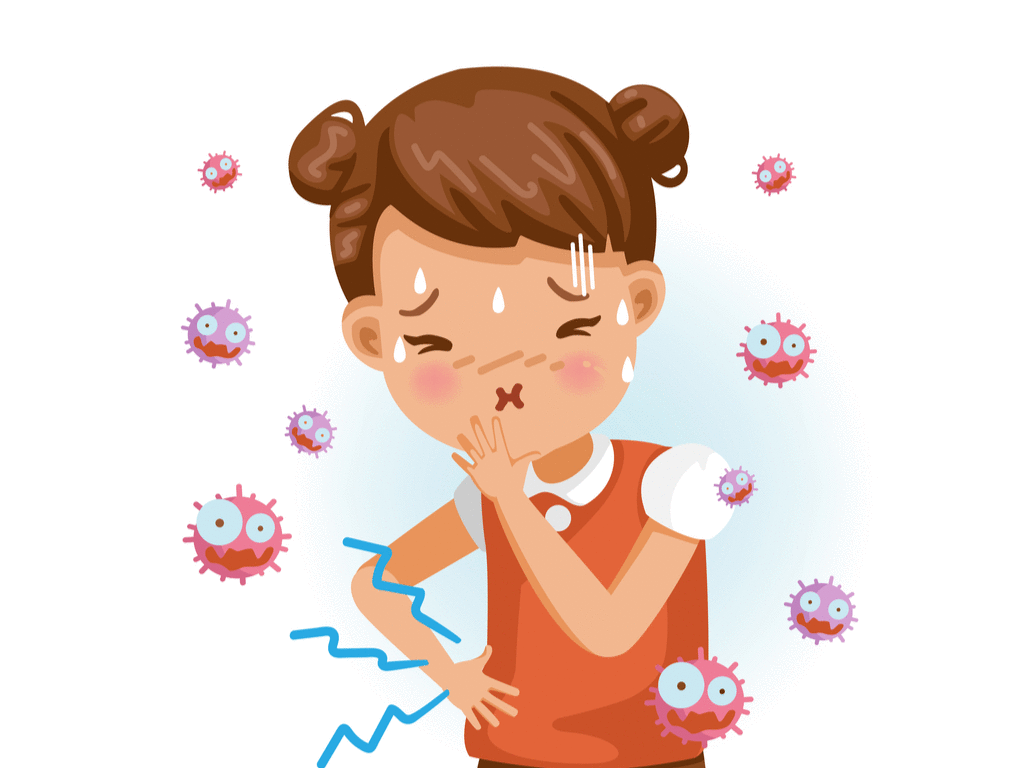
Acidity
In order to reduce the acidity content, we can drink water that the acids will be diluted in some cases. The need for antacid is a must that will neutralise the strong acid. We can take antacids such as milk of magnesia, which contains magnesium hydroxide.
Note: Human body works within the pH range of \(7.0\) to \(7.8\), and only in this narrow range of pH living organisms survive.
Agriculture:
In agriculture, the nature of the soil is important. The use of chemical fertilisers in excess causes the soil to become acidic. Plants cannot grow well in acidic soil. Hence, the soil is treated with bases like fast lime (calcium oxide) or slaked lime when it becomes too acidic (calcium hydroxide) to neutralise it. Similarly, burnt wood ashes in the soil can also reduce the acidic content present in the soil.
In agriculture, the nature of the soil is important. The use of chemical fertilisers in excess causes the soil to become acidic. Plants cannot grow well in acidic soil. Hence, the soil is treated with bases like fast lime (calcium oxide) or slaked lime when it becomes too acidic (calcium hydroxide) to neutralise it. Similarly, burnt wood ashes in the soil can also reduce the acidic content present in the soil.

pH effect on agriculture
Organic matter (compost) is added to the soil if it is too essential. Organic matter produces acids that help to restore the soil's basic nature.
Industries:
Acids such as sulfuric acid can be found in industrial effluents. Before being discharged into rivers and streams, it is treated by adding lime to neutralise it. Similarly, coal and other fossil fuels are burned to generate electricity in power plants.
When fossil fuels are burned, sulphur dioxide gas is released into the atmosphere as an acidic pollutant. As a result, power plants neutralise this acidic gas with powdered lime (\(CaO\)) or limestone (\(CaCO_3\)) to prevent air pollution.
Note: If the pH of rainwater is less than \(5.6\), it is called acid rain.

pH and environmental pollution
The factory wastes are neutralised by adding basic substances. Most factory wastages contain high acidic content; if it is released into the ecosystem, it will affect the organisms that live in that ecosystem.
Let us do an activity to check whether plants and animals are sensitive to pH:
Collect soil from your surroundings
Step 1: Add about \(2g\) soil in a test tube.
Step 2: Now, add \(5ml\) of water to the test tube containing soil.
Step 3: Mix the contents of the test tube.
Step 4: Filter the contents in the test tube using filter paper and collect the filtrate.
Step 5: Check the pH of this filtrate with the help of a universal indicator.
Step 6: Observe the colour change.
Observation: The pH paper can turned into red or blue depending on the nature of the soil.
Result: If the pH paper turns red, it means the soil is acidic in nature, whereas if it turns blue, then it is basic in nature.
We can conclude that the ideal pH content in the soil for plant growth should be around \(7\), which is neutral. Thus, neutral soil is the best for plants grown in any region.