PDF chapter test TRY NOW
When a single element displaces another element in a compound, this is known as a single displacement or exchange reaction.
A + BC → AC + B
\(Zn\) + \(CuBr_2\) → \(ZnBr_2\) + \(Cu\) (cation single replacement)
\(Cl_2\) + \(2KI\) → \(2KCl\) + \(I_2\) (anion single replacement)
Example:
Experiment \(2\)a: To study the chemical reaction of zinc with sulphuric acid.
Materials required:
-Zinc metal granules
-Dilute sulphuric acid
-Red and blue litmus papers
-Test tube and
-Candle
Step 1: Take few zinc granules in a test tube.
Step 2: Add about \(10\) mL of dilute sulphuric acid to zinc granule. Effervescence comes out from the reaction mixture, as shown in the below figure.
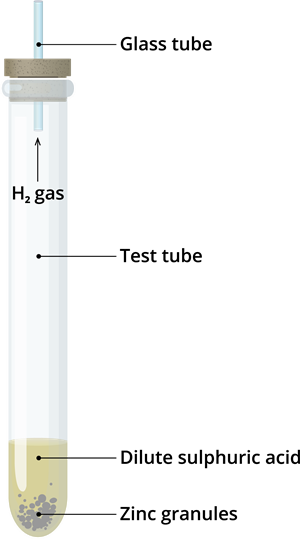
The reaction of zinc granules with dilute sulphuric acid
Step 3: Perform the tests as given in the observation table and record your observations.
Observation:
No. | Test | Activity | Observations |
1 | Colour | Look at the colour of the gas liberated | Colourless gas is evolved (effervescence). |
2 | Smell | Fan the gas gently towards your nose with your hand | Odourless gas is observed. |
3 | Litmus test | Bring moist blue and red litmus papers near to the mouth of the test tube | Does not change the colour of the litmus papers. |
4 | Combustion test | Bring a lighted candle near to the mouth of the test tube | The liberated gas produces a pop sound and turns off the candle. |
Result:
Zinc metal reacts with dilute sulphuric acid to produce zinc sulphate and hydrogen gas.
\(Zn\)(s) + \(H_2SO_4\)(aq) → \(ZnSO_4\)(aq) + \(H_2\)(g)
Zinc sulphate formation is an example of a single displacement reaction of a non-metal by a metal.
The observation from the above table clearly shows that the produced gas is hydrogen. It is the only gas with these properties; colourless, odourless, does not change the litmus paper (because it is neutral) and turns off the candle.
Final result:
From the above observation, the nature of the produced gas is neutral because it does not change the colour of the litmus paper.
Hydrogen is flammable. When hydrogen burns (or externally ignites) in the air to produce water and heat, this process is called combustion.
Hydrogen is flammable. When hydrogen burns (or externally ignites) in the air to produce water and heat, this process is called combustion.
Conclusion:
We already know what a single displacement reaction is. The above experiment clearly shows the single displacement reaction. Here zinc has displaced another element in a compound, sulphate, from dilute sulphuric acid.
