PDF chapter test TRY NOW
Redox reactions:
A reaction involving both oxidation and reduction simultaneously means one reactant gets oxidised, the other gets reduced. Such reactions are named as oxidation-reduction reactions or redox reactions.
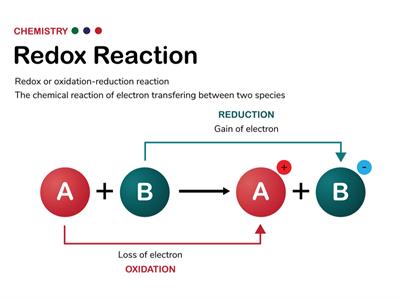
Oxidation is a-
-gain of oxygen
-loss of hydrogen
-loss of electrons
-an increase in oxidation number
-gain of oxygen
-loss of hydrogen
-loss of electrons
-an increase in oxidation number
Reduction is a-
-loss of oxygen
-gain of hydrogen
-gain of electrons
-a decrease in oxidation number
-gain of hydrogen
-gain of electrons
-a decrease in oxidation number
Let us perform an activity to see the process of oxidation and reduction.
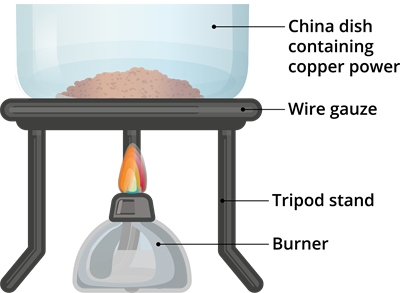
Activity \(1.11\): Oxidation and reduction reactions. Materials required:
-Copper powder
-China dish
-Burner
-Tripod stand and
-Wire gauze
Experimental procedure:
Step 1: Take \(1\)g of copper powder in the China dish.
Step 2: Put the tripod stand over the burner and the wire gauze on top of it.
Step 3: Now put the China dish containing copper powder over the wire gauze.
Step 3: Now put the China dish containing copper powder over the wire gauze.
Step 4: Turn on the burner and heat the China dish.
Step 5: Take the black copper(II) oxide powder and heat it again with hydrogen gas.
Observation:
- After heating, we observed that the copper powder becomes coated with black colour.
- This black coloured powder is copper(II) oxide.
- The black powder of copper(II) oxide turns brown.
Copper redox reaction
Result:
After heating, copper becames copper oxide because of oxidation; when a chemical reaction gains oxygen, it is said to be oxidised.
The black powder of copper(II) oxide turns brown again, and we get copper powder back. This reaction happens because of the reduction. When there is a loss of oxygen in a chemical reaction, it is said to be reduced.
Oxidation reaction:
\(2Cu\) + \(O_2\) \(2CuO\)
Redox reaction:
\(CuO\) + \(H_2\) \(Cu\) + \(H_2O\)

Conclusion:
In this activity, the copper(II) oxide is loses oxygen during the reduction reaction. The copper(II) oxide is reduced to copper. The hydrogen is gains oxygen, and is being oxidised. An oxidation-reduction reaction occurs when one reactant receives oxygen while the other reactant loses oxygen or when one reactant is oxidised while the other reactant is reduced at the same time. The oxidation-reduction reaction is also known as the redox reaction.
Example:
Here are some more examples of redox reactions:
\(ZnO\) + \(C\) → \(Zn\) + \(CO\)
\(MnO_2\) + \(4HCl\) → \(MnCl_2\) + \(2H_2O\) + \(Cl_2\).
In \(ZnO\) with \(C\), reaction carbon is oxidised to \(CO\) and \(ZnO\) is reduced to \(Zn\).
In \(MnO_2\) with \(4HCl\), reaction \(HCl\) is oxidised to \(Cl_2\) whereas \(MnO_2\) is reduced to \(MnCl_2\).
From the above examples, we state that in the process of oxidation, a substance gains oxygen or loses hydrogen during a reaction. Where as, in process of reduction a substance is reduced if it loses oxygen or gains hydrogen during a reaction.
Recall the activity \(1.1\), where a magnesium ribbon burns with a dazzling flame in the air (oxygen) and converts into a white substance, magnesium oxide. In this reaction is magnesium oxidised or reduced?
When magnesium reacts with oxygen (air), the following reaction takes palace.
\(Mg\) + \(O_2\) → \(MgO\)
In this reaction, magnesium \(Mg\) is oxidized to form \(MgO\) by the gain of oxygen. We know the gain of oxygen is oxidation, So, the above reaction is an example for oxidation.
