
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoMole Concept:
Wilhelm Ostwald coined the term "mole" around \(1896\), originating from the Latin word moles, which means "heap" or "pile." Any material may be considered as a set of atoms or molecules.

Wilhelm Ostwald
In \(1967\), the unit mole was adopted as a straightforward way to report a huge number–the vast heap of atoms and molecules in a sample.
One mole of any particles such as atoms, molecules, or ions is that quantity in number having a mass equal to its atomic or molecular mass in grams.
The one mole of any substance is a constant value. That is, the number of particles (atoms, molecules or ions) present in one mole of any substance is constant, with a value of .
This value is known as the Avogadro number or Avogadro constant, which is obtained experimentally. Avogadro number can be represented as .
Therefore, one mole of particles (atoms, molecule, or ion) \(=\)
Mole:
Relative atomic or molecular mass in grams is equal to the mass of one mole of a substance. The mass of one atom of an element is measured in atomic mass units by its atomic mass (u).
How to find the gram molecular mass or molar mass of a molecule?
To get the mass of one mole of an atom of that element (molar mass), we have to take the same numerical value by changing the units from \(‘u’\) to \(‘g’\).
Example:
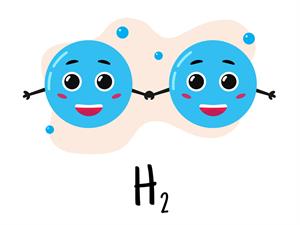
The atomic mass of hydrogen \(= 1u\). One g of hydrogen has one mole of atoms, which is equal to atoms of hydrogen.
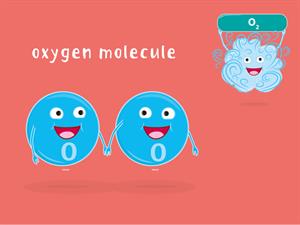
\(16u\) Oxygen has one atom of Oxygen. \(16g\)of Oxygen has one mole of atoms, which is equal to atoms of Oxygen.
Molecular Mass:
In atomic mass, we get the mass of the atom alone, but in the molecular mass, we get the mass of the total molecule.
Molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of a substance. It is, therefore, the relative mass of a molecule expressed in atomic mass units (u).
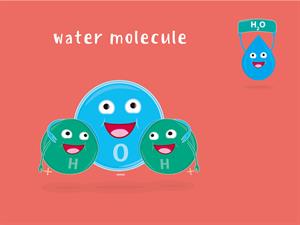
For instance, the molecular mass of \(H_2O\) is two times the mass of one H-atom plus the mass of one O-atom.
Formula unit mass:
For the substances whose constituent particles are ions, the formula unit mass is used. It is the number of the atomic masses of all the atoms in a compound's formula.

For example, unit mass of ionic \(NaCl\), is .
Note:To calculate or to find
1. Mass from a mole of molecule or atom - Use
2. Atomic mass from atomic number - Use , where n is
3. Number of particles from mass - Use
Mole