PDF chapter test TRY NOW
Matter changing its state due to pressure.
In the previous exercise, we have studied that the matter can change its state due to the effects of the temperature. But, what will happen if we change the pressure? If we start putting pressure and compress a gas enclosed in a cylinder,what will be consequences?
How will the matter will react due to change in pressure?
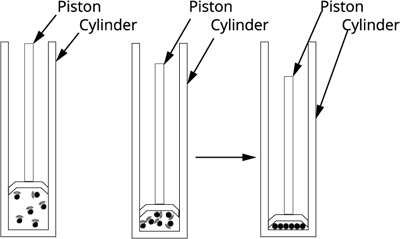
Effects on pressure
The effects of pressure are negligible on solids substances and more on liquid and most on the gases.

Effects on pressure
When the pressure is applied to a container of gas, the gas particles come together. When the pressure is increased that reduce the kinetic energy of the particle.
When pressure is increased further the gas-particle get near to each other, and then converted into the liquid form.
When pressure is increased further the gas-particle get near to each other, and then converted into the liquid form.
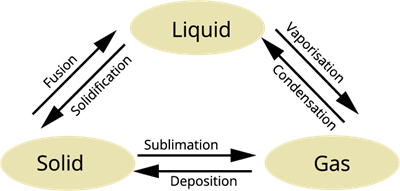
Interconversion of the three states of matter
Therefore, whenever we apply pressure to the gas particles, it converts its state to liquid.
Therefore, whenever we apply pressure to the gas particles, it converts its state to liquid.
