PDF chapter test TRY NOW
What is chemical properties?
A chemical property is a feature of a substance that may be seen when involved in a chemical reaction.
(i) Valence electrons:
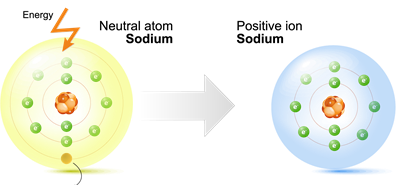
Sodium valence shell
Metal's atoms normally have one, two, or three electrons in their outermost (valence) shell.
(ii) Formation of ions:
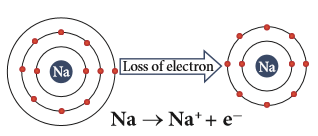
Sodium (positive) ion formation
Metals are electropositive as they lose electrons to produce positive ions.
(iii) Discharge of ions:
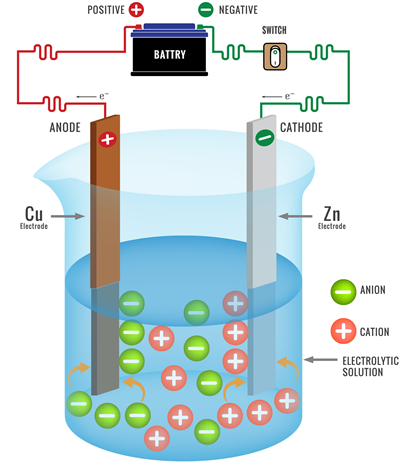
Electrolysis ions discharging
Metals are releases ions at the cathode during the electrolysis of their compounds.
(iv) Atomicity:

Vapour state monoatomic molecule
Metal molecules in the vapour state are generally monoatomic.
(v) Nature of oxides:
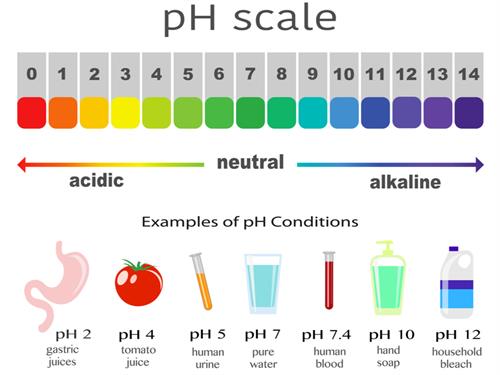
Metal oxides are usually basic (alkaline) in nature.
