PDF chapter test TRY NOW
Features of the Groups:
The vertical columns in the periodic table start from top to bottom, are called groups. The periodic table has \(18\) groups.
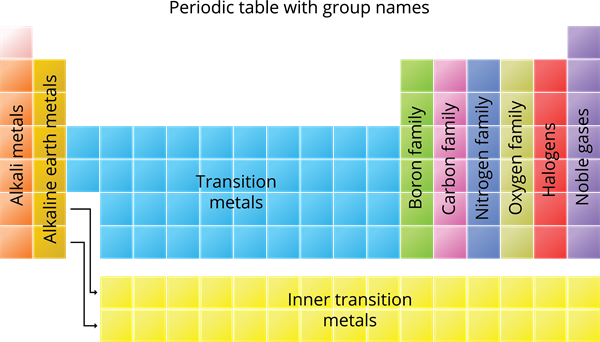
Group Number | Family |
\(1\) | Alkali Metals |
\(2\) | Alkaline earth metals |
\(3\) to \(12\) | Transition metals |
\(13\) | Boron Family |
\(14\) | Carbon Family |
\(15\) | Nitrogen Family |
\(16\) | Oxygen Family (or) Chalcogen family |
\(17\) | Halogens |
\(18\) | Noble gases |
- Lanthanides and Actinides are Group \(3\) elements, referred also as inner transition elements.
- Except for group \(18\), every element in each group has the same amount of electrons in its valence shell and has the same valency. All group \(1\) elements, for example, contain one electron in their valence shells (\(1s^1\) ). As a result, the valency of all alkali metals is one.
- Because the elements in a group have identical valence shell electronic configurations, they have similar chemical properties.
- The physical properties of the elements in a group vary gradually, such as melting point, boiling point, and density.
- The atoms of 'group \(18\)' elements have a stable electronic configuration in their valence shells and are thus unreactive.
Periodic trends in properties: Periodicity:
The electronic configurations of elements help us to describe the periodic recurrence of physical and chemical properties. Anything which repeats itself after a fixed interval is called periodic, and this behaviour is called periodicity. Some of the atomic properties of the elements are periodic.
Periodic properties:
Atomic radius, ionic radius, ionization energy, electronegativity, and electron affinity are called periodic properties.
The modern periodic table's primary significance is that it provides a fair conclusion of the common characteristics and trends within a group or a period, allowing us to predict the properties of any element with considerable accuracy, even if that element is unknown to us. Let's take a look at the periodic trend of some of these properties.
