PDF chapter test TRY NOW
Step (ii). Alumina electrolytic reduction - Hall’s Process:
The Hall-Héroult process is the principal industrial process for smelting aluminium. It includes dissolving aluminium oxide (alumina) (obtained most often from bauxite, aluminium's chief ore, for the Bayer process) in molten cryolite and electrolyzing the molten salt bath in a purpose-built cell. The Hall–Héroult process applied at an industrial scale happens at \(940\)-\(980\)°C and produces \(99.5\) - \(99.8\) % pure aluminium.
The electrolytic reduction of fused alumina (\(Al_2O_3\)) in the electrolytic cell produces aluminium.
An electrolytic reduction is a form of electrolysis. Electric current passes through an ionic substance in a molten or dissolved state, causing the electrodes to react chemically and the materials to decompose.
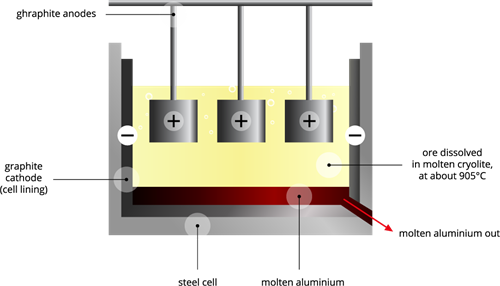
Cathode: Iron tank lined with graphite
Anode: A collection of graphite rods suspended in a molten electrolyte.
Electrolyte: Pure alumina + molten cryolite + fluorspar (fluorspar reduces the fusion temperature of electrolyte).
Temperature: \(900\) - \(950\)°C.
Voltage used: \(5\) - \(6\) V.
Overall reaction: \(2Al_2O_3\) → \(4Al\) + \(3O_2\)↑.
Voltage used: \(5\) - \(6\) V.
Overall reaction: \(2Al_2O_3\) → \(4Al\) + \(3O_2\)↑.
Hall's Process:
This electrolytic cell consists of an iron tank lined with a graphitic cathode, and anodes are suspended in a molten electrolyte. The electrolyte solution contains pure alumina \(Al_2O_3\), molten cryolite and fluorspar; we maintain the temperature around \(900\)-\(950\)°C. We apply \(5\)-\(6\) V of current through the electrodes. The alumina (Bayer processed) reduces at the cathode to form pure molten aluminium on the bottom of the iron tank.
Pure aluminium extraction (Hall-Héroult) process
Aluminium is deposited on the cathode; oxygen is released on the anode. Oxygen reacts with graphite (carbon) to form (CO_2\).
What is physical property?
A physical property is a property of matter that can be observed and measured without changing the sample's chemical identity.
Physical Properties of Aluminium:
(a). Aluminium is a silvery-white metal. (You can see in the kitchen)

Silvery-white aluminium
(b). Aluminium is light and has a low density (\(2.7\)).

Light and low, dense cans
(c). Aluminium is ductile and malleable. (Drawn into a thin wire and capable of being shaped)

Aluminium wire and pipe
(d). Aluminium is a good heat and electrical conductor. (A material that allows charge (current or heat) to flow in one or more directions).

Aluminium conducting heat and electricity
(e). The melting point of aluminium is \(660\)°C.

Aluminium melting process
(f). Aluminium can be polished to give it a lustrous, appealing appearance. (Shiny and bright appearance)

Shiny aluminium foil
