
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoUnder any given set of conditions, the amount of solute that can be dissolved in a given amount of solvent is limited.
Solutions are classified into the following types based on the amount of solute in the given solvent:
- Saturated solution
- Unsaturated solution and
- Supersaturated solution
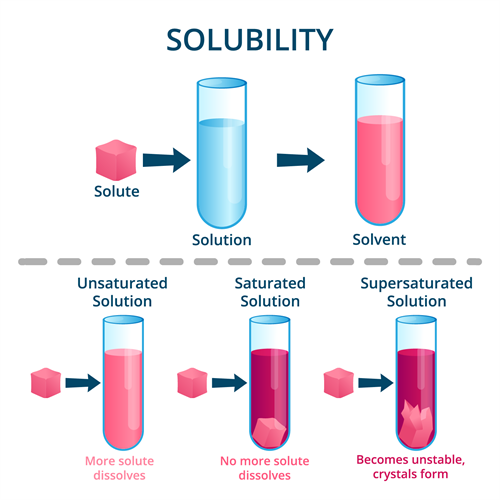
Solubility
Note: For scientific work, room temperature is assumed to be in the range of \(20 to 25 °C\).
A saturated solution is one in which no more solute can be dissolved in a specific amount of solvent at a given temperature.
Example:
At \(25° C\), \(36 g\) of sodium chloride in \(100 g\) of water forms a saturated solution. If some more sodium chloride is added after this, it will be undissolved.
At a given temperature, an unsaturated solution contains less solute than a saturated solution.
Example:
At \(25° C\), \(10 g\), \(20 g\), or \(30 g\) of sodium chloride in \(100 g\) of water forms an unsaturated solution.
Supersaturated solution:
At a given temperature, a supersaturated solution contains more solute than a saturated solution.
Example:
At \(25° C\), \(40 g\) of sodium chloride in (100 g\) of water forms a supersaturated solution. This state can be attained by adjusting any other conditions, such as temperature and pressure.
Supersaturated solutions are unstable. When the solution is disturbed, the solute reappears as crystals.
You are given two samples of solutions of \(NaCl\). Can you identify which one is saturated? And how?
If more solute is added and it still does not dissolve, then the original solution has become saturated. If the solute added gets dissolved, the original solution is unsaturated.