PDF chapter test TRY NOW
The majority of the substances are soluble in water. That is why water is referred to as a "Universal solvent."

Universal solvent
However, some substances do not dissolve in water. As a result, other solvents, such as ethers, benzene, and alcohols, are used to prepare a solution by dissolving the substances.
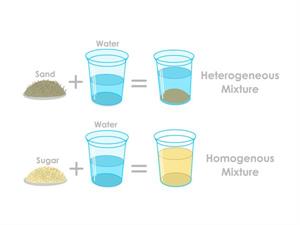
Some substances do not dissolve in water
Based on the type of solvent used solutions are classified into two types:
- Aqueous solutions
- Non-aqueous solutions
Aqueous solutions:
The aqueous solution is a solution in which water acts as a solvent.
Example:
Common salt in water, sugar in water, copper sulphate in water, and so on.

Dissolving salt in water
Here in the above examples, the solvent is water, and the solute is common salt, sugar, copper sulphate, etc.
Non-aqueous solutions:
The non-aqueous solution is a solution in which any liquid other than water acts as a solvent.
Example:
Iodine dissolved in carbon tetrachloride, sulphur dissolved in carbon disulphide, etc.

Iodine does not dissolve in an aqueous solution

Iodine dissolved in non-aqueous solution

(a) Sulphur dissolved in \(CS_2\)
(b) Sulphur insoluble in water
(b) Sulphur insoluble in water
Sulphur dissolved in carbon disulphide: The solvent is carbon disulphide, and the solute is sulphur.
