
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoCharles's Law:
Jacques Charles, a French scientist, formulated Charles's law in \(1787\). It is also known as the law of volume.
The statement of Charles's law is as follows:
The volume (\(V\)) of a given mass of a gas, at constant pressure (\(P\)), is directly proportional to its absolute temperature (\(T\)).
As a mathematical equation, Charles's law is written as either:
Where,
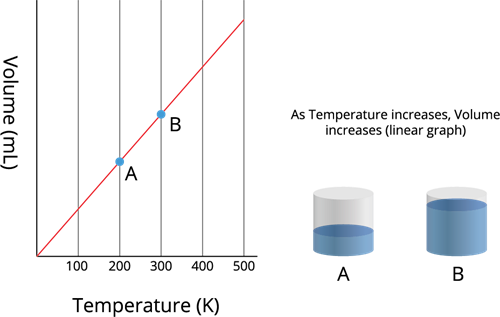
Charles's law - Temperature vs Volume
Another form of the equation (assuming there are two sets of conditions and setting both constants to each other) that might help solve problems is,
Where,
Avogadro's law:
Amedeo Avogadro proposed Avogadro's law in \(1811\).
The statement of Avogadro's law is as follows:
It states that the volume occupied by an ideal gas is directly proportional to the number of atoms or molecules of the gas present in the container at constant pressure and temperature.
As a mathematical equation, Avogadro's law is written as either:
Where,
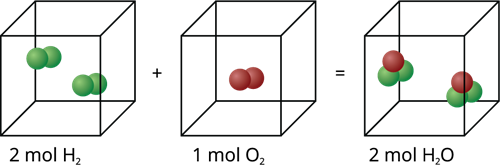
Molecular addition
Another form of the equation (assuming there are two sets of conditions and setting both constants to each other) that might help solve problems is,
Where,