
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn the previous sections, we have learnt about the effects of heating on solids. In this section, we are going to look at,
What happens while heating a liquid?
When a liquid is heated, the energy of the atoms in a liquid or gas increases, and they are forced further apart. The amount of expansion in liquids and gases vary from substance to substance depending on their properties.
In a liquid, particles will flow or slide over one another but stay near the vessel's bottom. The bonds between separate molecules are usually less.
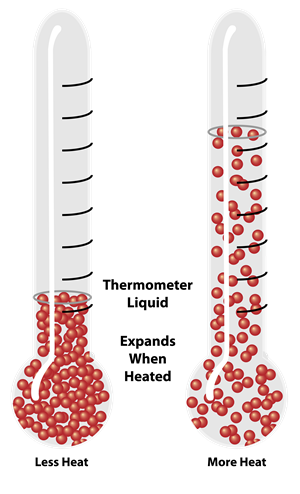
Expansion in liquid due to heating
When the substance is heated, its particles gain energy, which is exerted as kinetic energy. The particles in the substance move around faster, weaken the intermolecular forces of attractions, and are thus held less closely together. The liquid expands.
The expansion in liquid higher than a solid for the same temperature rise. A gaseous substance has the maximum expansion when compared with solid and liquid.
The coefficient of cubical expansion of liquid does not depend on its temperature, whereas its value for gases mainly depends on the temperature of gases.
Whenever liquid is heated, it is done by keeping liquid in vessel and applying heat energy to the liquid through the vessel.
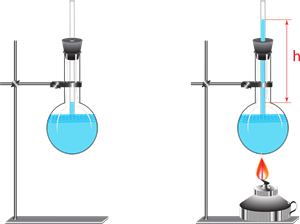
Experiment to understand the expansion in liquid due to heating
The thermal energy applied will be partly used to expand the vessel and partly expand the liquid. Thus, what we observe may not be the actual or real expansion of the liquid.
Hence, for liquids, we can define,
- Real expansion and
- Apparent expansion