PDF chapter test TRY NOW
Soap and detergent are water-soluble compounds, i.e. they dissolve in water quickly.
The saponification process between the sodium or potassium salts with the fatty acids makes up soaps for cleansing purposes.
Surface tension: This property is based on cohesive forces that exist between the liquid molecules. The molecules present in the liquid undergo a net inwards force with no force acting above the surface—causing the surface molecules to contract and resist breaking.
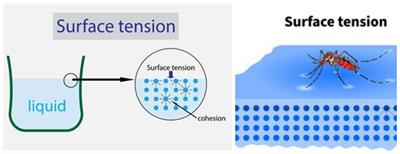
Surface tension
Hydrophilic group (head) - water-loving
Hydrophobic group (tail) - water repellent
Hydrophobic group (tail) - water repellent
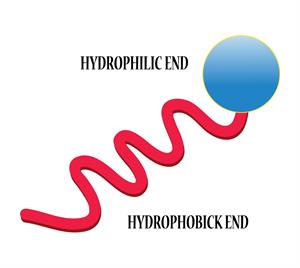
The hydrophilic and hydrophobic end
The hydrophilic and hydrophobic groups in the soap molecules react with the water molecules and cleanse the dirt away. The dirt mostly contains oil which is insoluble in water.
The long-chain carbons present in the soap makes the hydrophobic group which attracts the oil present in the dirt, and the sodium molecule acts as the hydrophilic group, which is water-loving. This formation is known as micelles.
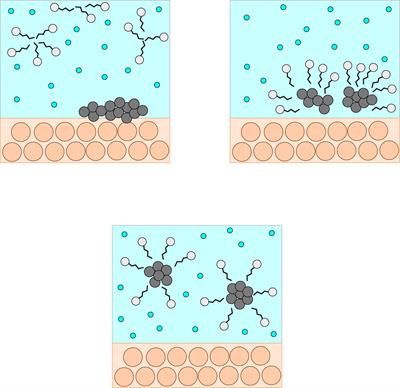
Cleansing action
In this image, we can see the head and the tail, which cleanses the dirt away. These micelles act in cleaning the dirt from the fabric.
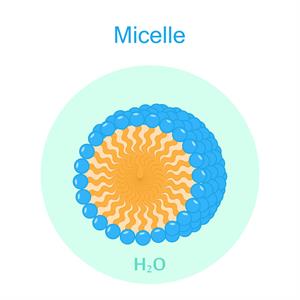
\(3d\) image of the micelles
This image shows the action of micelles cleaning the dirt from the scalp and hair. Similarly, this type of cleansing action takes place in shampoo, floor cleaners, hand wash etc.
