PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWhat is matter?
Matter is all around us. In other hand, it can be defined as anything that has mass and occupies space. The air you are breathing is also a matter. See some examples given below:




Matter consists of atoms. Atom is the smallest particle that we cannot see through nacked eyes, even on the standard microscopes. Science introduced a technology to identify the structure of atoms: SEM (Scanning Electron Microscope) and TEM (Tunneling Electron microscope) that uses electricity to map atoms.
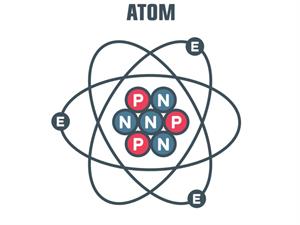
An atom consists of particles of neutron (N), electron (E) and protons (P) in which neutrons and protons are present in the nucleus of the atom and electrons are present outside of the nucleus.
An atom consists of particles of neutron (N), electron (E) and protons (P) in which neutrons and protons are present in the nucleus of the atom and electrons are present outside of the nucleus.
The matter is made up of tiny particles. For example, water is made up of small water particles that one drop of water has water particles, and a single dot made by pen has more than \(2\) Lacs of particles.
Characteristic of the particles of matter:
- The sizes of particles are very small.
- They attract each other (the attractive force is different for a different form of matter).
- Particles move constantly.
- They have space between them; however, the spacing may vary for a different form of matter.
Physical states of matter:
Based on the characteristic of particles in matter, it can be classified into three states.
- Solids
- Liquids
- Gases
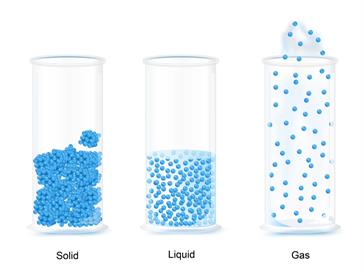
Particles in solids:
Particles in the solids are tightly packed with no space between them. Hence, it has a definite shape and volume. Examples: Stone, granite and limestone.
Particles in liquids:
Particles in the liquids are arranged randomly with some space between them. Hence, it has a definite volume but not shape.
Particles in the solids are tightly packed with no space between them. Hence, it has a definite shape and volume. Examples: Stone, granite and limestone.
Particles in liquids:
Particles in the liquids are arranged randomly with some space between them. Hence, it has a definite volume but not shape.
Example: Water
Particles in gases:
Particles in the gases are arranged far away. Hence, it has no definite shape and volume. It can move fast and colloid one another.
Particles in gases:
Particles in the gases are arranged far away. Hence, it has no definite shape and volume. It can move fast and colloid one another.
Example: Air
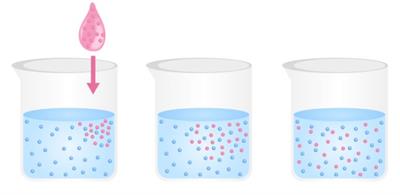
Diffusion:
Diffusion is the tendency of particles to spread out in order to occupy the available space.
In other words, diffusion is the movement of particles from a higher concentration to a lower concentration.
Diffusion occurs in liquids and gases since they can move easily.