
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIf all elements are made up of the same type of electrons, protons and neutrons, what is the difference between the different atoms of different elements like carbon and iron?

To find the solution for this, scientists led further investigations to discover the reason. Finally, they found that the number of protons inside the nucleus of an atom determines what element it is. For example, if an atom's nucleus has only one proton, then such kind of atoms are hydrogen atoms. If there are eight protons in the nucleus, then that atom is oxygen.
Atomic number:
- The number of electrons or protons present in an atom is called the atomic number.
- It is represented by the letter '\(Z\)'. If we know the atomic number of an atom, we can easily find the number of electrons or protons present.
- The hydrogen nucleus has only one proton, which revolves around one electron. It means that the atomic number of hydrogen is one(\(Z\)=\(1\)).
- There are two protons and two electrons in the orbit around the nucleus of helium atom. Therefore, the atomic number of helium is two(\(Z\)=\(2\)).
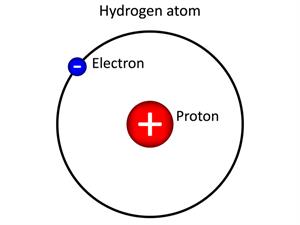
In the above image, the hydrogen atom has one proton, and an electron revolves around it. Thus, the atomic number of a hydrogen atom is one.
Mass number:
- We have studied that the mass of an atom is concentrated in its nucleus because the size of the electron is negligible.
- A mass number of an atom is equal to the sum of the number of protons and neutrons present in the nucleus.
Atomic mass or mass number = Number of protons + Number of neutrons
- Lithium atom contains three protons and four neutrons.
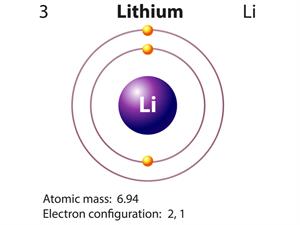
Atomic mass or mass number of Lithium (\(A\)) = \(3\)+\(4\) = \(7\).
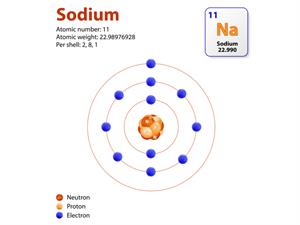
In a sodium atom, there are \(11\) protons and \(12\) neutrons. Hence, its atomic mass or mass number(\(A\)) = \(11\) + \(12\) = \(23\).
Isotopes:
Atoms that have the same atomic number but with different mass numbers are called Isotopes. Example: Hydrogen(), Deuterium(), Tritium().
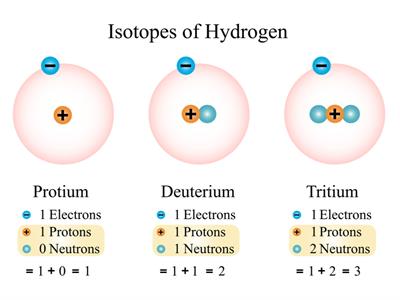
Isotopes of Hydrogen
Isobars:
The atoms with the same mass number but with the different atomic number are called Isobars.
Examples: Calcium-\(40\) and Argon-\(40\)