PDF chapter test TRY NOW
We know that the particles in liquids are loosely packed with a weak intermolecular force of attraction. When heated, the particles vibrate at their position, and some break out from the weak force, causing evaporation or vaporisation. Evaporation or vaporisation is a slow process.
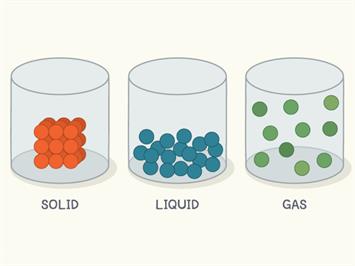
Arrangement of particles in matter
There are two types of vaporization: Evaporation (natural) and Boiling (heating). The only difference between evaporation (liquid to gas) and vaporisation (solid or liquid to gas) is the state from which the change occurs.
Both vaporisation (boiling) and evaporation are different because vaporisation is the bulk process, whereas evaporation is the surface phenomenon.
1. Evaporation:
Evaporation
- When the substance from the liquid state is boiled, it changes into a vapour. The change of state from liquid to vapour is known as evaporation. Evaporation occurs below the temperature of the boiling point of the substance.
- During evaporation, the liquid's temperature falls and to maintain the temperature, and it absorbs the heat from the surroundings.
- The evaporation process speeds up when the surface area is large compared to the smaller surface area. This process occurs at the upper surface of the liquid.
- The increase in temperature, wind speed and decreased level of humidity speeds up the process of evaporation.
Factors affecting the process of evaporation:
- Impurities present in liquid makes the process slower. For example, take two glasses. One should be filled with water with no impurities, and the other should be impure (mix water with salt or sugar). Place both the glasses in sunlight for hours and check the level of water present in them. We could see that the glass containing only water would have evaporated faster than that of water mixed with impurities.

Salt formation
- The liquid evaporates faster when the temperature is hot; if not, the process will occur at a slower rate. Coldwater evaporates slower compared to hot water.

Water evaporates from the kettle.
- If the humidity (water vapour in the air) is higher, then evaporation is slower. We can see that clothes dry faster in summer (low humidity) than in winter (high humidity).

- The wind (moving air) speed also determines the rate of evaporation. On a windy day, the clothes dry up faster.

Clothes drying

Drying meat
The brine (concentrated salt solution) is formed in the sea due to the evaporation of water. Similarly, drying clothes, hair, meat, fish etc., in sunlight is due to the evaporation process.  Boiling is a physical change as there is only a change in the state of the substance. This is the process of vaporisation.
Boiling is a physical change as there is only a change in the state of the substance. This is the process of vaporisation.
2. Boiling:

Boiling of vegetables in water
The process where liquid changes into a vapour state on heating is known as boiling process.
On heating the liquid continuously, the particles present in the liquid vibrate faster making the particles to move and break the forces that keep them together. This causes the liquid to change into a vapour.
Substances change their state when the temperature reaches the liquid's boiling point, causing it to change into a vapour.
Factors that affect the rate of boiling:
Pressure:

Pressure cooker
For example, the pressure cooker increases the boiling point of water because the vapour formed here is not escaped, which causes an increase in pressure, due to which the boiling point is increased.
Impurity:

Salt increases the boiling point
The presence of impurities increases the boiling point of the substance. While cooking food, the salt added for taste increases the boiling point of water and makes the food cook.
The process of boiling and evaporation is a physical change that can be reversed. It is an endothermic type process that absorbs heat from its surroundings.
