
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoJohn Dalton:
1. John Dalton was born to a poor weaver's family in England in \(1766\). At the age of twelve, he started his career as a teacher. He became a school principal seven years later.

John Dalton
2. Dalton moved to Manchester in \(1793\) to teach mathematics, physics, and chemistry at university.
3. He taught and did study there for the majority of his time. He proposed his atomic theory in \(1808\), which was a watershed moment in the study of matter.
4. John Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
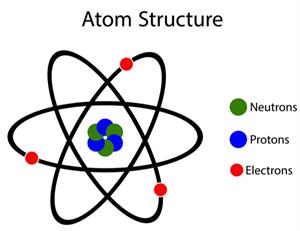
According to John Dalton, all matters, whether an element, a compound or a mixture, are composed of tiny particles called atoms.
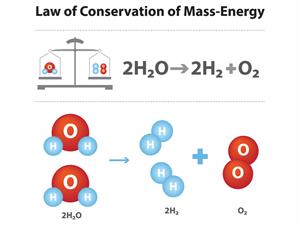

Atoms can neither be created nor destroyed.
The following postulates are proposed by John Dalton:
- Atoms are the tiniest particles that comprise all matters.
- In a chemical reaction, atoms are indivisible particles that cannot be formed or destroyed.
- A given element's atoms have the same mass and chemical properties.
- The masses and chemical properties of atoms in various elements vary.
- Compounds are formed when atoms combine in small whole-number ratios.
- In a given compound, the relative number and type of atoms remain constant.
Dalton's atomic theory
We got an idea about an atom from Dalton's atomic theory, but first of all, we should know what an atom is. In the next lesson, we will explore more about the atom.