PDF chapter test TRY NOW
Crookes Experiment:
In 1878, William Crookes discovered visible rays travelling between two metal electrodes when experimenting with a discharge tube. These rays are called Crookes rays or Cathode rays.
Crookes tube is more commonly known as Cathode Ray Tube (CRT).
- A long glass tube filled with gas and sealed at both ends is known as a cathode ray tube.
- It is constructed of two metal plates (which serve as electrodes) connected by a high-voltage wire.
- The cathode is the electrode that is attached to the battery's negative terminal (negative electrode).
- The anode is the electrode that is attached to the positive terminal (positive electrode).
- There is a side tube with a pump attached to it. The pump reduces the discharge tube pressure.
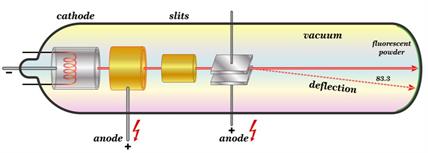
Cathode Ray Tube
Cathode Ray Tube:
Electrical discharge:
As electricity travels through air, it extracts electrons from gaseous atoms, resulting in cations' formation. This is known as an electrical discharge.
Electrical discharge
