
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAs you might be aware, food is critical for our survival as well as that of many other living beings. 
Have you ever seen your mother preparing food for you?

She uses the stove to boil rice, cook vegetables, and heat gravy, and to prepare many other dishes. When enough heat is applied, chemical reactions occur, converting raw (uncooked) food into cooked food.
Example:
In the laboratory, we can conduct a chemical reaction that requires the use of heat:
Case 1: In a dry test tube, heat a small amount of lead nitrate gently over a flame. Keep a close eye on the shifts.
You will hear cracking noises and see reddish-brown coloured gas (nitrogen dioxide) evolve.
Case 2: In the same way, limestone rocks are heated in factories to produce quicklime (calcium oxide).
Thus, the above two cases are an example of a chemical reaction carried out with the help of heat.
The preparation of common salt, drying wet clothes, meat, fish, hair in the sun, ironing of cloth are some examples of evaporation.


Evaporation is a simple example of a chemical reaction that takes place with the help of heat.
When the substance from the liquid state is boiled, it changes into a vapour. The change of state from liquid to vapour is known as evaporation.
Example:
Let us now check the evaporation with small activity by separating the coloured component with the blue or black ink:
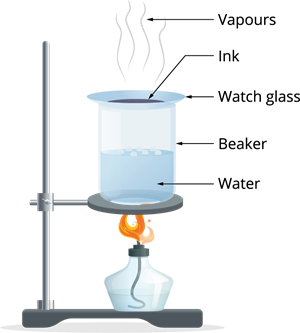
Step 2: After some time, the water in the beaker starts boiling. The heat is transferred to the watch glass, and evaporation takes place.
Step 3: Continue the process of heating until no changes seen on the watch glass.
Observation: The ink kept in the liquid form evaporates, leaving over the coloured component in the watch glass. This shows the ink is a mixture of water with dye.
Result: We can distinguish the volatile portion (solvent) from its non-volatile solute using the evaporation process.
As a result, certain chemical reactions can be achieved simply by the application of heat. Thermochemical reactions or thermolysis are the name for these types of reactions.