PDF chapter test TRY NOW
Chemical change: Any substance that changes its chemical properties is known as chemical change. In this change, a new substance is formed, and these changes are irreversible in nature. These changes are permanent. For example, photosynthesis reaction, burning of a matchstick, paper etc.
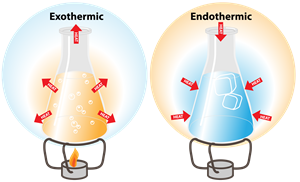
1. Exothermic change:
- The heat is released in this reaction
- Heat is the product
Combustion, condensation, freezing, neutralization reaction, dissolving an acid in water are few examples of exothermic changes as heat is released for the change to occur.
This is due to the bond formation, which in turn releases heat. The formation of bonds require less energy.
Example:
Combustion:

Heat is released
The burning of wood and paper releases heat energy to their surroundings, which is the best example of exothermic chemical change. The product formed here is different from the reactants. This is an irreversible change.
2. Endothermic change:
- The heat is absorbed in this reaction
- Heat is the reactant
Cooking, photosynthesis, evaporation are few examples of endothermic changes as the heat is absorbed for the change to occur.
Heat is absorbed as the breaking of bonds requires more energy in these reactions.
Example:
Cooking:


Heat is absorbed
Making dosa from batter is the best example as heat is absorbed from the pan, making the batter turn into the dosa. Similarly, making vada is also an example of endothermic chemical changes.
What is enthalpy?
Enthalpy: The change in heat energy during the reaction is known as enthalpy. The value of enthalpy differs with heat.
In case if heat is absorbed, the enthalpy is positive. The enthalpy is negative if heat is released.
Exothermic reaction:
Endothermic reaction:
Exothermic and endothermic reaction
