PDF chapter test TRY NOW
As we studied earlier, electrons (negative electric charges) in the outermost orbit of an atom can be easily removed. They can be transferred from one body to another. The body which gains electrons becomes negatively charged, and the body which loses electrons becomes positively charged.
Transfer of charges takes place in the following ways,
- Transfer by Friction
- Transfer by Conduction
- Transfer by Induction
Transfer by Friction:
- When a comb is rubbed with hair, the comb gains electrons from the hair and becomes a negatively charged body.
- Those electrons are accumulated on the surface of the comb.
- When a piece of paper is torn into bits, positive and negative charges are present at the bits' edges.
- Negative charges present in the surface of the comb attract positive charges in the paper bits. So, the paper bits move towards the comb.
- Therefore, rubbing certain materials with one another forms the electrical charges on the surfaces. From this, it is clear that charges are transferred by friction.

Hair and comb

Comb attracts paper bits
- A similar effect can be seen when we rub a few materials with one another. When a glass rod is rubbed with a silk cloth, the free electrons in the glass rod are transferred to the silk cloth.
- It is because the free electrons in the glass rod are less tightly bound as compared to that is in silk cloth.
- Since the glass rod loses electrons, it has a deficiency of electrons and acquires a positive charge. But, the silk cloth has an excess of electrons. So, it becomes negatively charged.
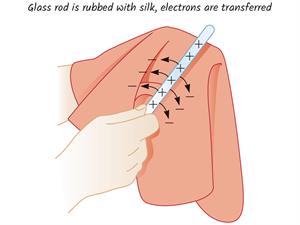
The glass rod rubbed with silk
The Glass rod is rubbed with a silk cloth, the free electrons in the glass rod are transferred to the silk cloth. When an ebonite rod (rod made by vulcanized rubber) is rubbed with fur, the fur transfers electrons to the ebonite rod. The electrons in the outermost orbit of the atoms in fur are loosely bound compared to the ebonite rod. The ebonite rod with excess electrons becomes negatively charged, and the fur with a deficiency of electrons is positively charged.
When rubber is rubbed with wool, electrons are transferred from wool to rubber. This electron transfer creates the deficiency of electrons in the wool and an excess of electrons in the rubber. Thus the wool acquires a positive charge, whereas rubber acquires a negative charge.
