PDF chapter test TRY NOW
Osmoregulation:
The balancing of water content in the body is known as osmoregulation.
What is osmolality?
This can be defined as, the concentration of the solutes in the solution per kilogram.
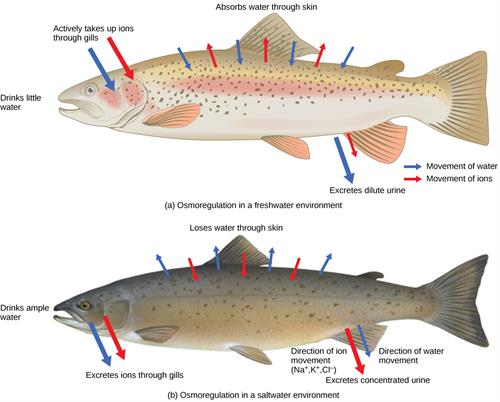
Osmoregulation in fishes
- Osmoregulation in freshwater fishes - In freshwater fishes, salt concentration is higher inside than outside, so usually, they will lose the salt and absorb water. So, they urinate in large amount compared to the marine fishes.
- Osmoregulation in marine fishes - In this type, the fishes have less amount of salt inside than the outer environment. So, they lose water and takes salt. And they will urinate a minimal amount.
There are two types:
1. Osmoconformers: These organisms usually tries to compensate the osmolality with the environment.
2. Osmoregulators: Usually, these types of organisms maintain their internal osmolality to overcome the very different external environment, and this will be by physiological processes.
Important!
Do you know?
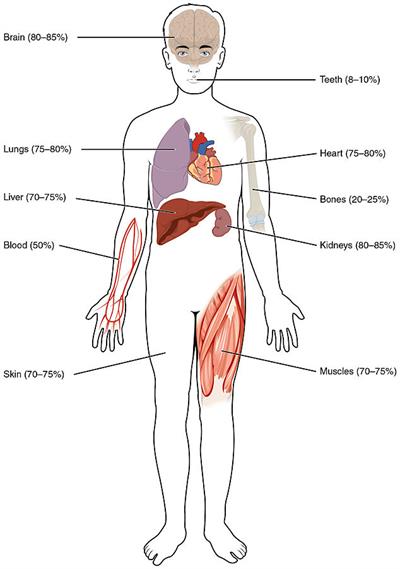
The amount of water in human body as follows:
Respiration:
The process of breaking down the stored energy glucose into the active energy (ATP) is called respiration .
Respiration occurs inside the cell in two places — one in the cytoplasm and the second in mitochondria.
Based on the location it occurs, respiration is two types:
- Aerobic respiration - Breaking down of glucose in the presence of oxygen, that occurs in both cytoplasm and mitochondria. The food is completely oxidized into \(H_2O\) and \(CO_2\) and releases more ATP with the presence of oxygen. The formula follows,
Glucose + Oxygen \(\rightarrow\) Carbon dioxide + Water + Energy
\(C_6H_{12}O_6\) + \(6CO_2\) \(\rightarrow\) \(6CO_2\) + \(6H_2O\) + \( ATP\)
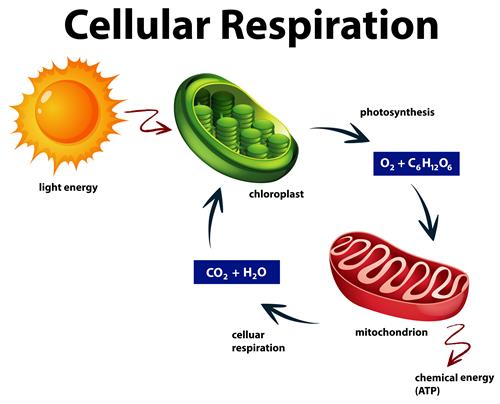
Aerobic respiration or cellular respiration
- Anaerobic respiration - Breaking down of glucose without the oxygen, which occurs only in mitochondria. The food is partially oxidized into ethanol or lactic acid with very little ATP without the presence of oxygen. This process is also known as fermentation. The formula follows,
Glucose \(\rightarrow\) Ethanol + Energy
\(C_6H_{12}O_6\) \(\rightarrow\) \(2C_2H_5OH\) + \(2CO_2\) + \(2ATP\)
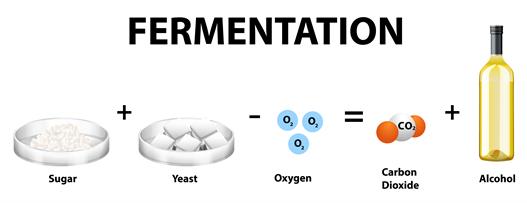
Anaerobic respiration or fermentation
Important!
Do you know?
In the human body, the end product of anaerobic respiration, lactic acid, is produced in muscle tissues due to the lack of oxygen. The oxygen depletion is mainly caused for athletes who does the heavy works. Lactic acid production leads to muscle spasm temporarily and will be resolved after enough oxygen is reached to the muscle. The formula is follows:
Glucose \(\rightarrow\) Lactic acid + Energy
\(C_6H_{12}O_6\) \(\rightarrow\) \(2C_3H_6O_3\) + \(2ATP\)
Difference between aerobic and anaerobic respiration:
Functions | Aerobic | Anaerobic |
| Reactants | Glucose + Oxygen | Glucose |
| Combustion | Complete | Incomplete |
| Energy yield | Gross - \(38 ATP\) Net - \(36 ATP\) | \(2 ATP\) |
| Products | \(CO_2\) and \(H_2O\) | \(C_3H_6O_3\) |
| Location | Cytoplasm & mitochondria | Cytoplasm |
Reference:
https://commons.wikimedia.org/wiki/File:Figure_41_01_02ab.jpg
https://commons.wikimedia.org/wiki/File:2701_Water_Content_in_the_Body-01.jpg
https://www.vecteezy.com/vector-art/455549-diagram-showing-cellular-respiration
https://www.vecteezy.com/vector-art/1424977-alcoholic-fermentation-chemical-equation
