PDF chapter test TRY NOW
Scientists in the \(17\)th century tried to figure out how to change one material into another. They made some broad generalisations based on their chemical change research. These generalisations are known as laws of chemical combination. They are as listed below:
- Law of conservation of mass
- Law of constant proportions
- Law of multiple proportions
- Law of reciprocal proportions
- Gay Lussac’s law of gaseous volumes
You have learned the first two of these five laws in \(8\)th grade. Let us recall them.
Law of conservation of mass
Matter neither be created nor destroyed, but it can be converted from one form to another.
Law of constant proportions
The same elements are always combined in the same proportion by mass in a chemical compound.
Law of multiple proportions
As two elements combine to form more than one compound, the mass of one combines with a fixed mass of the other in a small whole number ratio.
John Dalton proposed this law in \(1804\).
Nitrogen combines with oxygen to form two different oxides: Nitric oxide (\(NO\)) and Nitrogen dioxide (\(NO_2\)). The ratio of masses of oxygen in \(NO\) and \(NO_2\) for a fixed mass of nitrogen is \(1\) : \(2\).
Mass of Nitrogen (in g) | Mass of Oxygen (in g) | Mass of \(O\) in \(NO\) to mass of \(O\) in \(NO_2\) | |
\(NO\) \(NO_2\) | \(14\) \( \) \(14\) | \(16 \) \( \) \(16\) | \(1\) : \(2\) |
Similarly, the ratio of mass of \(O\) in \(SO_2\) to the mass of \(O\) in \(SO_3\) is \(2\) : \(3\), and the ratio of mass of \(O\) in \(CO\) to the mass of \(O\) in \(CO_2\) is \(1\) : \(2\).
Law of reciprocal proportions
When two different elements combine separately with the same weight of a third element, their mass ratios are either the same or a simple multiple of their mass ratios if they combine among themselves.
Jeremias Ritcher proposed this law in \(1792\).
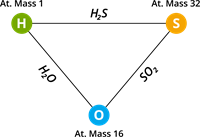
Law of reciprocal proportions
Here, hydrogen and oxygen combine separately with the same weight of carbon to form methane (\(CH_4\)) and carbon dioxide (\(CO_2\)), respectively.
Compounds | Combining elements | Combining weights |
\(CH_4\) | \(C\) \(H\) | \(12\) \(4\) |
\(CO_2\) | \(C\) \(O\) | \(12\) \(32\) |
Ratio of the different mass of hydrogen and oxygen that combines with the same mass of carbon \(=\) \(4\) : \(32\) or \(1\) : \(4\) ……(i)
Now, hydrogen and oxygen combine to form water (\(H_2O\)).
The ratio of mass of hydrogen to oxygen \(=\) \(2\) : \(16\) (or) \(1\) : \(8\) …...(ii)
The ratio obtained from (i) and (ii) is the same as the ratio obtained from the first. As a result, the reciprocal proportion law is explained.
