
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoCharacteristics of Carbon: The number of carbon compounds known presently is more than \(5\) million. Many newer carbon compounds are being separated or synthesised every day. Though the abundance of carbon is less, the number of carbon compounds alone is more than the number of combinations of all the elements used together. Why is it that carbon is the only element that has this property? It is because of the following unique features.
Catenation:
Catenation binds an element to itself or other elements through covalent bonds to form an open or closed chain compound. Carbon is the most common element which undergoes catenation and forms long-chain compounds. The carbon atom repeatedly links to itself through a covalent bond to form a linear, branched-chain or ring structure.
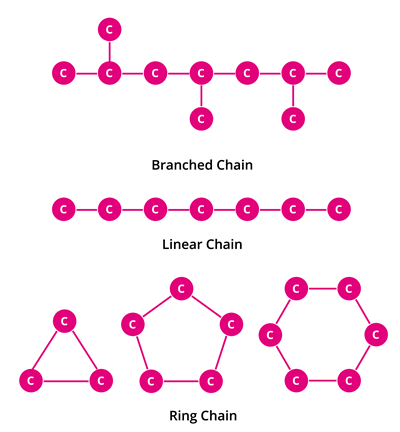
This property of carbon itself is the reason for the presence of many organic carbon compounds. So organic chemistry essentially deals with catenated carbon compounds.
For example, Starch and Cellulose contain chains of hundreds of carbon atoms. Even plastics we use in our daily life are macromolecules of catenated carbon compounds.
Another varied nature of carbon is its tetravalency. Carbon (Atomic number:\(6\)) the electronic configuration is \(2\), \(4\). Thus, it has four electrons in its outermost shell. According to Octet Rule, carbon needs four electrons to achieve the nearest noble gas (Neon) electronic configuration. So carbon tends to give its four electrons with other atoms to fill its octet. Thus it is termed as tetravalency. Likewise, carbon can make four covalent bonds with other elements.
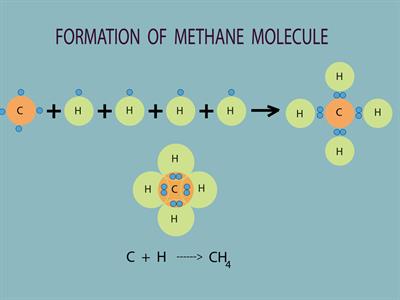
For example, in methane, a carbon atom gives four valence electrons with four hydrogens to form four covalent bonds and hence tetravalent.
Multiple Bonds: As seen above, the tetravalent carbon can make four covalent bonds.
Carbon can join with other elements or other carbon through the single, double and triple bonds with this tetravalency. As we all know, the nature of a compound's bonding is the primary factor that defines its physical and chemical properties. So, the strength of carbon to form multiple bonds is the main reason for various classes of carbon compounds.
The table below shows one of such classes of compounds called 'Hydrocarbons' and the type of bonding.
Type of bond | Example | Class of the compound |
| Single Bond | 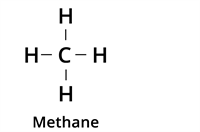 | Alkane |
| Double Bond | 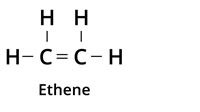 | Alkene |
| Triple Bond | 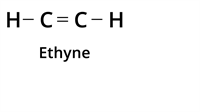 | Alkyne |
When one or more hydrogen atoms in hydrocarbons are restored by other elements such as oxygen (O), nitrogen (N), sulphur (S), halogens, and so on, different compounds with different functional groups are formed. You will learn more about them in your advanced class.