PDF chapter test TRY NOW
Lewis Dot Structure: When atoms join to make compounds, their valence electrons participate in bonding. As a result, having a method to depict the valence electrons in atoms is beneficial.
Lewis dot structure can be accomplished using the Lewis dot symbol method.
The Lewis dot structure or electron-dot symbol for an atom takes the form of the element symbol covered by dots indicating the electrons in the atom's valence shell.
The unpaired electron in the valence shell is denoted by a single dot, whereas a pair of dots denote the paired electrons. Other symbols, such as crosses or circles, may distinguish the electrons of the various atoms in the molecule.
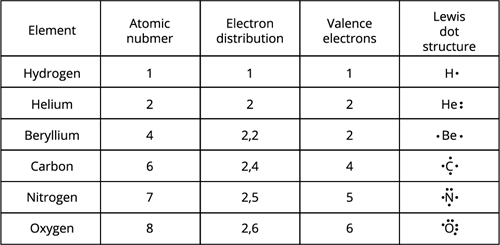
A chemical bond is the force of attraction between atoms that combines them into a unit known as a molecule.
Types of chemical bonds: All the elements have various valence shell electronic configurations. So how they combine to form compounds also differs. Therefore, there are various types of chemical bonding possible between atoms that make the molecules. Depending on the type of bond, they show various characteristics or properties. So types of bonding, considered to exist in molecules, are described below. Among these, let us learn about the Ionic, Covalent, and Coordinate bonds in this chapter and other types of bonds in the higher classes.
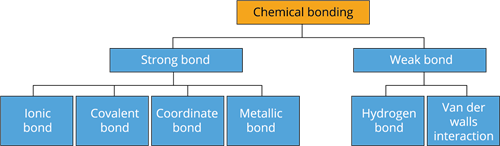
There are three types of chemical bonds:
- Ionic (or) Electrovalent bond
- Covalent bond
- Coordinate bond
