
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoFeatures of ionic compounds:
The primary factor that defines the properties of compounds is bonding between the atoms of a molecule. As a result, in ionic compounds, the atoms are held together by a strong electrostatic force, giving the compounds the following characteristics:
Physical state: A strong electrostatic force makes these compounds between cations and anions arranged in a well-defined geometrical pattern. Thus, ionic compounds are crystalline solids at an average temperature.

Salt is an example of a crystalline solid
Electrical Conductivity:
Ionic compounds are solid crystals, and so their ions are tightly bound together. As a result, the ions cannot move freely, and they do not conduct electricity in a solid-state. However, in a molten state, their aqueous solutions conduct electricity.
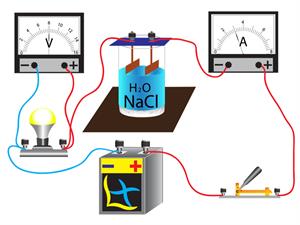
Electrical conductivity of the salt solution
Melting point:
The strong electrostatic interaction between the cations and anions binds the ions together, requiring a large amount of energy to separate them. So ionic compounds possess high melting and boiling points.

Steel melting
Solubility:
Water and other polar solvents make ionic compounds soluble. Ionic compounds are insoluble in benzene and non-polar solvents.

Sugar soluble in tea
Density, hardness and brittleness:
Ionic compounds include a high density, and they are pretty hard because of the strong electrostatic force between the ions. But, they are highly brittle.

Various nature of metal
Reactions: Ionic compounds go through ionic reactions that are almost immediate and fast.