PDF chapter test TRY NOW
Let us see the difference between elements and compounds with few examples.
Elements:

Elements cannot be further separated (Gold, Silver)
The substances which cannot be broken down to the simpler form of substances is known as the elements.
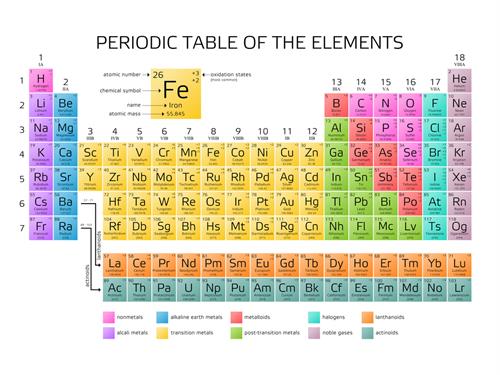
The elements are made of only one kind of atom and elements are the fundamental particle.
Example:
Oxygen, nitrogen, helium, hydrogen.
Compounds:

Compounds can be separated
The substances have fixed components and can be broken down into elements by chemical reactions.
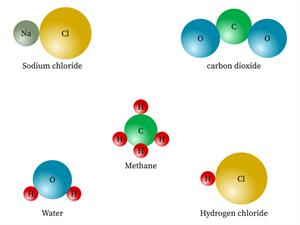
But, the compounds are made of more than one kind of element.
Example:
Sugar, water, salt, alcohol, baking soda.
Let us see some of the elements:
Pure Substance | Column I | Column II |
| Argon \(Ar\) | Element | Cannot be separated |
| Lead \(Pb\) | Element | Cannot be separated |
| Phosphorous \(P_4\) | Element | Cannot be separated |
| Neon \(Ne)\ | Element | Cannot be separated |
| Sulphur \(S_8\) | Element | Cannot be separated |
Example:
Let us see some of the elements present in the compounds:
1. 

Chemical name: Water
Chemical formula: \(H_2O\)
Elements: Hydrogen, Oxygen.
Number of different elements: \(2\)
2. 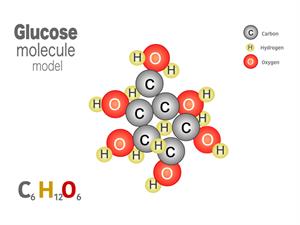

Chemical name: Glucose (Sugar)
Chemical formula: \(C_6H_{12}O_6\)
Elements: Carbon, Hydrogen, Oxygen.
Number of different elements: \(3\)
3. 

Chemical name: Calcium oxide
Chemical formula: \(CaO\)
Elements: Carbon, Oxygen.
Number of different elements: \(2\)
4. 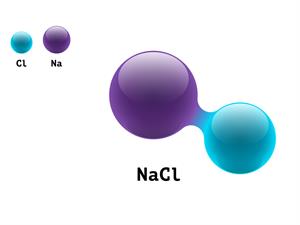

Chemical name: Sodium chloride (salt)
Chemical formula: \(NaCl\)
Elements: Sodium, Chlorine.
Number of different elements: \(2\)
5. 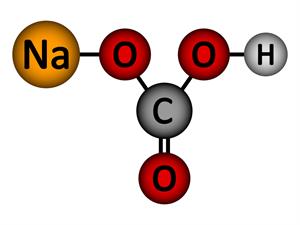

Chemical name: Sodium bicarbonate (Baking soda)
Chemical formula: \(NaHCO_3\)
Elements: Sodium, Carbon, Hydrogen, Oxygen.
Number of different elements: \(4\)
6. 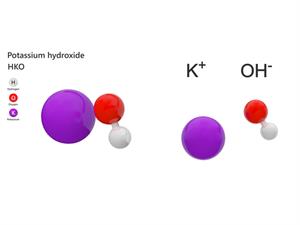

Chemical name: Potassium hydroxide
Chemical formula: \(KOH\)
Elements: Potassium, Oxygen, Hydrogen.
Number of different elements: \(3\)
In the above examples, the chemical names such as water, glucose, calcium oxide, sodium chloride, sodium bicarbonate and potassium hydroxide are called compounds. We can separate these compounds into their elements through chemical methods.
Let us now see the difference between these elements and compounds:
Elements | Compounds |
| There is only one kind of atom in it. | It's made up of a variety of atoms. |
| An atom is the smallest particle in the element which maintains all of its properties. | The molecule is the smallest particle in the compound which maintains all of its properties. |
| Not possible to break it down into simpler elements. | Chemical methods are used to break it down into its constituent elements. |
