PDF chapter test TRY NOW
Solutions: A solution is a mixture of two or more substances that appears to be uniform in appearance. A solution is a homogeneous mixture of two or more substances.

Solutions
A solution is a mixture in which one substance dissolves fully in the other.
A solute is a part of a solution that is present in a smaller quantity by weight. A solvent is a variable that is present in a greater quantity by weight.
If we add the solvent and solute, we get the solution.
Solution \(=\) Solvent \(+\) Solute.
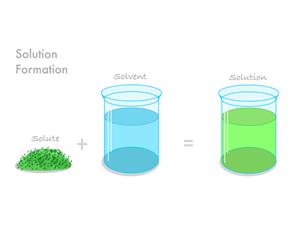
Formation of solution
Types of solutions based on the size of solute particle:
- True solution
- Colloidal solution
- Suspensions
Example:
Let us look at the activity to find out the type of solution:
Activity 1: To find a true solution
Step 1: Add one spoon of sugar or salt to a glass full of water and stir it well.
Step 2: Keep it aside for \(10\) minutes.
Activity 1: To find a true solution
Step 1: Add one spoon of sugar or salt to a glass full of water and stir it well.
Step 2: Keep it aside for \(10\) minutes.

Salt mixed with water
Observation: We can observe that we get a clear solution and the particles never settle down.
Result: The observed solution is known as the true solution.
Activity 2: To find a colloidal solution
Step1: Add one spoon of starch or chalk to a glass full of water and stir it well.
Step 2: Keep it aside for \(10\) minutes.

Chalk mixed with water
Observation: We can observe a cloudy mixture.
Result: The observed solution is known as a colloidal solution.
Activity 3: To find a suspension solution
Step1: Add one spoon of wheat flour to a glass full of water and stir it well.
Step 2: Keep it aside for \(10\) minutes.

Wheat flour mixed with water
Observation: We can observe that we get a turbid mixture and small particles sink at the bottom after some time.
Result: The observed mixture is called suspension.
Note:
True solution: The particle size is less than \(10^-7\) cm.
Colloidal solution: The particle size is between \(10^-7\) cm and \(10^-5\) cm.
Suspension: The particle size is greater than \(10^-5\) cm.
Colloidal solution: The particle size is between \(10^-7\) cm and \(10^-5\) cm.
Suspension: The particle size is greater than \(10^-5\) cm.
