PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoLaw of Octaves:
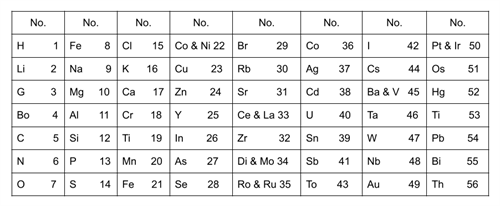
In \(1866\), John Newlands established \(56\) known elements in increasing order of their atomic mass.
When elements are organised in the order of increasing atomic masses, the properties of the eight-element (starting from a given element) is a repetition of the properties of the first element, which is known as the law of octaves.
The octave's first and last notes are the same for the Indian music system, sa, re, ga, ma, pa, da, ni, sa. Similarly, in Newlands' table of octaves, the element \(F\) is eighth from the element \(H\), implying that they have similar properties.
Newland’s table of octaves:
Drawbacks of Newlands law of octaves:
- There have been occasions when two elements, such as cobalt and nickel, were fitted into the same slot.
- Some elements, totally different in their properties, were fitted into the same group. (Halogens are grouped with metals like cobalt, nickel and platinum.)
- The law of octaves did not apply to elements with atomic masses greater than calcium.
- Newlands' table was limited to \(56\) elements and did not allow for the addition of new ones.
- Since inert gases had not yet been discovered, they were not included in the periodic table.
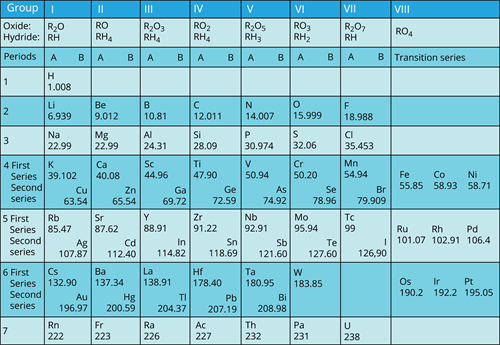
Mendeleev’s periodic table:

In \(1869\), Russian chemist Dmitri Mendeleev noted that similar properties repeat at frequent intervals when the elements are arranged in their atomic masses.
The chemical and physical properties of elements are the periodic functions of their atomic masses.
Features of Mendeleev’s periodic table:
- Mendeleev classified the periodic table into seven horizontal rows (called periods) and eight vertical columns (called groups).
- Each group divided into two sub-groups.
- The first time the elements with similar properties are grouped.
- Scientists found certain elements to be unable to be placed in their proper groups in this manner, due to their wrong atomic mass value. Later, the problem of atomic mass value has been resolved in the modern periodic table.
- Columns were left empty to accommodate unknown elements at the time, and their properties were predicted.