PDF chapter test TRY NOW
We have seen the reaction of acid and base with metals. Let us now see how the acids react with metal carbonates and metal hydrogen carbonates with the help of an experiment.
Important!
Caution: The experiment should be carried out in the presence of a teacher.
Example:
Activity 1:
To study the reaction of an acid with metal carbonates and metal hydrogen carbonates.
Materials required:
- Sodium carbonate \(Na_2CO_3\)
- Sodium hydrogen carbonate\(NaHCO_3\)
- Calcium hydroxide solution \(Ca(OH)_2\)
- Test tubes
- Delivery tube
- Lid or a cork
Step 1: Take two test tubes and mark them as A and B.
Step 2: Take \(0.5g\) of sodium carbonate \(Na_2CO_3\) in test tube A and \(0.5g\) of sodium
hydrogen carbonate or sodium bicarbonate \(NaHCO_3\) in test tube B.
Step 3: Add \(2mL\) of dilute hydrochloric acid in both the test tubes.
Step 4: Place a cork to the mouth of each test tube and insert the delivery tube into it.
Step 5: Take calcium hydroxide solution in a separate test tube and leave the free end of the delivery tube inside the test tube containing calcium hydroxide solution or lime water.
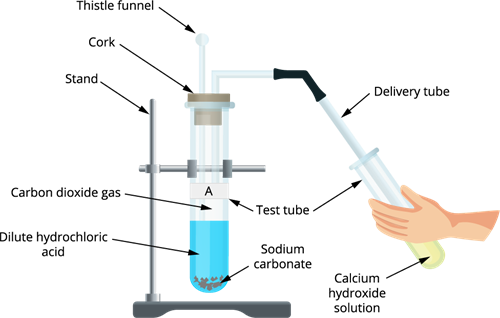
Apparatus setup for the reaction
Observation: Gas bubbles are formed in both the test tubes.
Result: Carbon dioxide is produced from the reaction, which turns the lime water milky.
Let us now see the chemical equation for the above experiment:
1. \(Metal carbonate + Acid\rightarrow Salt + Water + CO_2\)
Test tube A: \(Na_2CO_3 + 2HCl \rightarrow 2NaCl + H_2O + CO_2\)
2. \(Metal hydrogencarbonate + Acid\rightarrow Salt + Water + CO_2\)
Test tube B: \(NaHCO_3 + HCl \rightarrow NaCl + H_2O + CO_2\)
On passing the gas formed from the above reaction (carbon dioxide) through the lime water, we get the following chemical reaction:
\(Ca(OH)_2 + CO_2 \rightarrow CaCO_3 + H_2O\)
Thus, the limewater turns milky due to the formation of calcium carbonate\(CaCO_3\).
The excess of carbon dioxide formed reacts with calcium carbonate as given below, forming calcium bicarbonate or calcium hydrogen carbonate \(Ca(HCO_3)_2\), soluble in water.
\(CaCO_3 + H_2O + CO_2 \rightarrow Ca(HCO_3)_2\)
Therefore, we can conclude that all the metal carbonates and metal hydrogen carbonates react with acid forming their respective salt along with carbon dioxide and water.
We know metal carbonates and metal hydrogen carbonates are basic in nature, so they react easily with acids forming salt, water and carbon dioxide.
Since metal carbonates and metal hydrogen carbonates are considered as bases that can neutralise acids, they do not react with the base again as there will be no reaction.
