
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoExample:
With the help of activity let us now see if crystals of salts really dry:
Step 1: Take a dry boiling tube.
Step 2: Add few crystals of copper sulphate (solid form) in a dry boiling tube.
Step 3: Heat the crystals in the boiling tube.
Step 4: Observe the colour of copper sulphate crystals after heating.
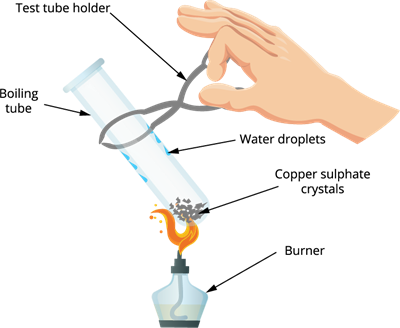
Salt on heating
Observation: The colour of copper sulphate crystals is changed from blue to white, and we can also see drops of water inside the boiling tube.
Dry copper sulphate crystals contain water of crystallisation. So when we heat the crystals, this water is removed, and the salt turns white.
Step 5: Add two to three drops of water to the sample of copper sulphate obtained after heating and observe the changes.
Result: Copper sulphate solution turns into blue colour again from white when water is added.
What is the water of crystallisation?
The water of crystallisation is the fixed number of water molecules present in one formula unit of salt. For example, hydrated copper sulphate \(CuSO_4. 5H_2O\) contains five water molecules in one formula unit of copper sulphate.
The water of crystallisation is the fixed number of water molecules present in one formula unit of salt. For example, hydrated copper sulphate \(CuSO_4. 5H_2O\) contains five water molecules in one formula unit of copper sulphate.
a) We know the chemical formula for baking soda. What does the \(10 H_2O\) signify?
b) Does it make \(Na_2CO_3\) wet?
Answer:
a) Ten water molecules in one formula unit of baking soda. This denotes the water of crystallisation.
b) No.
Gypsum:
Gypsum is another salt that contains water of crystallisation. It has two water molecules as crystallisation water. Gypsum \(CaSO_4.2H_2O\) is found in several forms and is of great economic importance.

Gypsum crystal
It is the hydrated form of calcium sulfate, which is of great industrial importance. Its chemical name is calcium sulfate dihydrate.
Occurrence:
The swordlike transparent selenite gypsum crystals \(2m\) or more are often found at the Cave of Swords, Mexico. These are mostly found in sedimentary rocks along with minerals such as halite, borax, sulfur, calcite and dolomite.

Gypsum salt
These occur in extensive beds formed by the evaporation of ocean brine(highly concentrated salt solution). These are alteration products of sulfides in ore deposits and volcanic deposits.
Properties of Gypsum:
It is colourless or white. But it is often tinted brown, grey, yellow, green, or orange due to impurities. It is moderately soluble in water, but when the temperature increases, the solubility decreases.
This character contradicts to other salts as the solubility of calcium sulfate in water is an exothermic process (liberation of heat takes place).
Gypsum Uses:
Gypsum Uses:

Used as a binder in fast-dry tennis court clay
- It is used in construction as wallboard, drywall, sheetrock or plasterboard.
- It is used in concrete blocks in building construction.
- It is used in Plaster ingredients.
- It is used as a binder in fast-dry tennis court clay.
- In making of dietary calcium as a source.
- It is a common ingredient in making mead (an alcoholic beverage created by fermenting honey with water).
- In shampoos, foot creams and many hair products.
- They are used in mushroom cultivation to stop grains from clumping together.