PDF chapter test TRY NOW
Activity \(1.3\): The experiment aims to see the evolution of a gas and change in temperature.
Important!
CAUTION: Handle the acid with care.
Materials required:
-Zinc granules
-Hydrochloric acid
-Conical flask or test tube and
-Beaker
Experimental procedure:
Step 1: Take some zinc granules in a conical flask or a test tube.
Step 2: Take some hydrochloric acid into the beaker.
Step 3: Add this hydrochloric acid to the conical flask or a test tube with zinc granules.
After adding HCl to Zinc granules:
Observation:
(i). Bubbles of gas start liberating from the conical flask.
\(Zn\) + \(2HCl\) → \(ZnCl_2\) + \(H_2\) gas starts liberating.
(ii). The conical flask felt hot from outside (flask became hot).
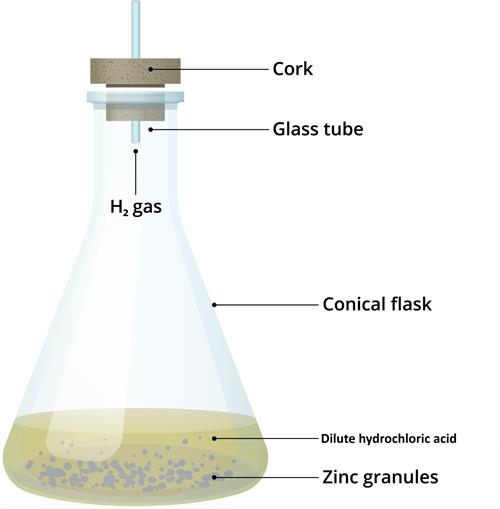
Evolution of a gas and change in temperature.
Results:
Zinc granules combine with hydrochloric acid to form zinc chloride and hydrogen gas.
\(Zn\) + \(2HCl\) → \(ZnCl_2\) + \(H_2\)
Conclusion:
We already know that the change in temperature and the evolution of gas are also part of chemical reactions. This activity clearly shows the chemical reaction (temperature change and gas evolution), which means the zinc chloride formation, and hydrogen evolution reactions lead to a hot flask.
We may noticed several chemical reactions taking place around us when we observe the changes around us. We will study the various types of chemical reactions and their symbolic representation in the upcoming sections.
