PDF chapter test TRY NOW
In solution or molten form, morereactive metals can displace less reactive metals from their compounds is known as displacement reaction.
All metals are not equally reactive in nature as we learnt in the previous sections. The reactivity of various metals with air, water and acids was investigated. However, not all metals react with these reagents. As a result, we were unable to arrange all of the metal samples in decreasing order of reactivity. The displacement reactions discussed in Chapter \(1\) provide further evidence for metal reactivity. It is simple and easy; if metal X displaces metal Y from a solution, X is more reactive than Y.
Let us do the experiment to better understanding.
- Take a clean copper wire and an iron nail.
- Take two test tubes and label them 'A' and 'B'.
- Take a copper sulphate solution in test tube A and an iron sulphate solution in test tube B.
- Immerse the copper wire in an iron sulphate solution and the iron nail in a copper sulphate solution in test tubes (as shown in the picture).
- Allow them to stand for \(20\) minutes.
- We observe that the reaction occurs in test tube A, where iron displaced copper from copper sulphate, while no reaction occurs in test tube B.
- The more reactive metal displaces the less reactive metal in such a reaction.
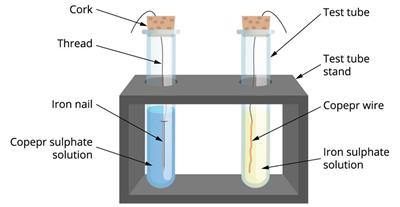
The experiment for the displacement reaction.
Iron displaced copper from copper sulphate
Image credits: CBSE_Metals and Non-metals_Page.No. \(45\)
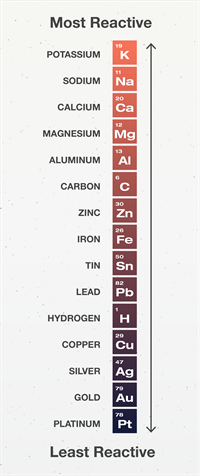
Reactivity Series
The reactivity series is a collection of metals arranged in decreasing order of activity.