PDF chapter test TRY NOW
Based on their chemical properties, elements can be categorized as metals and non-metals. When non-metals dissolve in water, they generate acidic oxides. On the other hand, most metals form basic oxides.
Examples for acidic oxides: \(SO_2\), \(CO_2\), \(P_2O_3\), \(P_2O_5\), etc.
Activity 1: To find the chemical properties of metals.
- Take some sulphur powder and a magnesium ribbon.
- The magnesium ribbon should be set on fire. Collect and dissolve the resultant ashes in water.
- Red and blue litmus paper were used to test the resultant solution.
Is the product generated when magnesium is burned acidic or basic?
Answer: Basic - Burn the sulphur powder now. To collect the gases created by the burning sulphur, place a test tube over it.
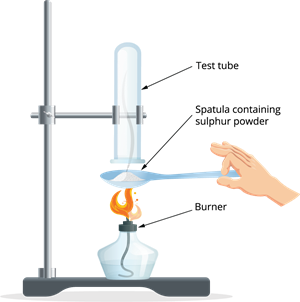
Burning sulphur powder on a spatula
- Fill the test tube with water and shake it.
- Use blue and red litmus paper to test this solution.
- Is the sulphur-burning product acidic or basic in nature?
Answer: Acidic
The equations for the above reactions are as follows:
Reaction with oxygen or air
Activity 2: To observe the chemical properties of substances when burnt in the air.
- The teacher's help is required for the next exercise. It would be preferable if students used safety glasses.
- With a pair of tongs, hold any of the samples taken before and try to burn them over a flame. Repeat with the other metal samples.
- If the product has been formed, collect it.
- Allow time for the items and the metal surface to cool.
- Which metals are the most flammable?
Answer: Magnesium - When the metal burned, what colour flame did you see?
Answer: Blue - What is the appearance of the metal surface after it has been burned?
Answer: Silver white - Arrange the metals in order of decreasing reactivity toward oxygen.
Answer: \(Na > Mg > Al > Zn > Fe > Pb > Cu\) - Are the products water soluble?
Answer: Aluminium oxides, copper oxides, iron oxides, lead oxides, magnesium oxides, and zinc oxides are insoluble. Sodium oxide is soluble in water.
Almost all metals react with oxygen resulting in the formation of metal oxides. These metal oxides are basic in nature. However, the reactivity of metals differ towards oxygen.
| Temperature | Reaction with oxygen (\(O_2\)) |
| At room temperature | |
| At slight temperature | |
| At high temperature |
Metal oxides are basic. However, some metal oxides, such as aluminium oxide, zinc oxide, etc., can act as acidic and basic. Such metal oxides are called amphoteric oxides. They produce salts and water when they react with acids and bases.
Example: The reaction of aluminium oxide with both acid and base.
The majority of metal oxides are water-insoluble. However, some metal oxides, such as sodium oxide and potassium oxide, dissolve in water to form alkalis.
Reaction rates
The rate of reaction between metals and oxygen is not the same for all metals. The reactivity of various metals towards oxygen differs. Metals like potassium and sodium react so rapidly with oxygen and readily catches fire in the air. They cannot be kept in water since they react exothermically in water. As a result, they are immersed in kerosene oil to protect them and prevent accidental fires.
On the other hand, metals such as magnesium, aluminium, zinc, lead, etc., have a thin layer of oxide on their surfaces at room temperature. The metal is protected from further oxidation by the protective oxide layer.
Iron does not burn when heated, but iron filings do when put into the burner's flame. Copper does not burn, but it does leave a black film of copper (II) oxide on the hot metal.
Silver and gold do not combine with oxygen even at high temperatures.
We have observed that sodium is the most reactive metal among the others. On the other hand, the reaction of magnesium is less vigorous, implying that it is not as reactive as sodium.
Do you know?
Anodising is the process of forming a thick oxide layer of aluminium. When aluminium is exposed to air, a thin oxide layer forms. The aluminium oxide coating protects it from additional corrosion. The resistance can be increased even more by thickening the oxide layer. A clean aluminium piece is used as the anode and electrolysed with dilute sulphuric acid during anodising. The anode's oxygen gas reacts with the aluminium to form a thicker protective oxide coating. This oxide coating can be easily coloured to give aluminium items an attractive design.
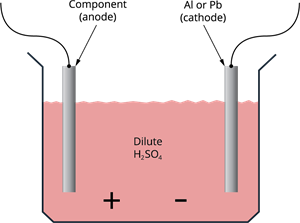
Anodising process
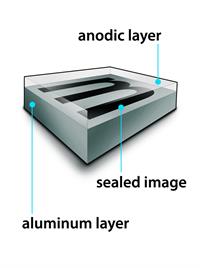
Anodised aluminium
Reference:
https://th.bing.com/th/id/R.3d04214dcb45a0cfc474e6be85d82b9a?rik=%2fAXuAm0QOGpifQ&riu=http%3a%2f%2ftrenchpress.com%2fwp-content%2fuploads%2f2021%2f01%2fShort-Guide-to-Hard-Anodising.png&ehk=W5Ki8ieoKVy%2fCHGN0fpaVm3ZhNCWqehlqj4kGNl%2buhA%3d&risl=&pid=ImgRaw
