
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe say a material or substance is soluble in water when it completely dissolves in a liquid.
The term "Insoluble material" refers to a substance that does not dissolve.
Step 1: Take a spoonful of sugar and a glass of water.
Step 2: Take some sand and a water-filled glass.
Step 3: Mix the sugar and sand with the water in their respective glasses.
Step 4: Stir it for a few minutes and observe what is happening.
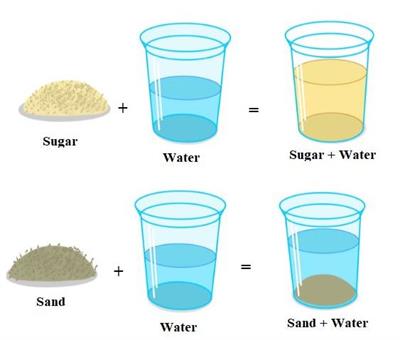
We can see that the sugar has disappeared or dissolved in water completely. On the other hand, the sand does not dissolve in water. As a result, the sugar substance is soluble, while the sand is referred to as an insoluble substance.
Example for soluble substance: Sugar, salt, washing powder, tablets
Example for insoluble substance: Chalk powder, sand, sawdust
Now, we have a better understanding of soluble and insoluble after performing solids experiments.
What about liquid? Do liquids dissolve in water?

Yeah, liquids dissolve in water, but not all liquids.
Let us now conduct one experiment to decide which liquids dissolve in water and which do not.
Step 1: Collect some lemon juice, vinegar, kerosene and coconut oil samples.
Step 1: Collect some lemon juice, vinegar, kerosene and coconut oil samples.
Step 2: Fill four vessels with half-filled water.
Step 3: Pour the four liquids into each of the four vessels separately.
Step 4: Stir it and let it be for a few minutes.
We can now see that the bottle containing lemon juice and vinegar has disappeared or dissolved in water. On the other hand, coconut oil and kerosene did not dissolve.
Step 4: Stir it and let it be for a few minutes.
We can now see that the bottle containing lemon juice and vinegar has disappeared or dissolved in water. On the other hand, coconut oil and kerosene did not dissolve.
As a result, we will tabulate the findings from the previous two tasks.
S.No | Solids & liquids | Mix well/Does not mix |
1 | Sugar/Salt | Mix well |
2 | Sand | Does not mix |
3 | Vinegar | Mix well |
4 | Lemon juice | Mix well |
5 | Coconut oil | Does not mix |
6 | Kerosene | Does not mix |
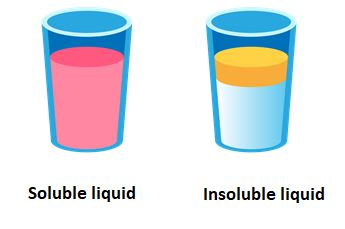
Now, we know that some liquids completely dissolve in water, others do not mix with water; and when left alone for a while, they form a separate layer as shown below:
So far, we have experimented on solids and liquid to find soluble and insoluble with water. But, what about gas?
Does any gas dissolve in water?


Some gases are water-soluble, while others are not. Some gases are normally dissolved in water in small amounts. For example, oxygen gas dissolved in water is essential for the survival of aquatic animals and plants.
Do you know?
Soda water is nothing but carbon dioxide dissolved in water under pressure. This is called ‘Aerated water’.

Reference:
https://www.flickr.com/photos/sfe-co2/31094457718