
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe learned that neutralisation is a process where the acidic and basic substance reacts quantitatively, which will neutralise the solution. In our daily lives, we use to do many neutralisation processes knowingly, and now we cover some of those neutralisation processes.
1. Indigestion:
Our stomach secretes hydrochloric acid \(HCl\), which helps the digestion process. Every morning and every time when we feel hungry, the stomach secretes this acid. But, if the secretion is high, then it causes indigestion which is not good for health.
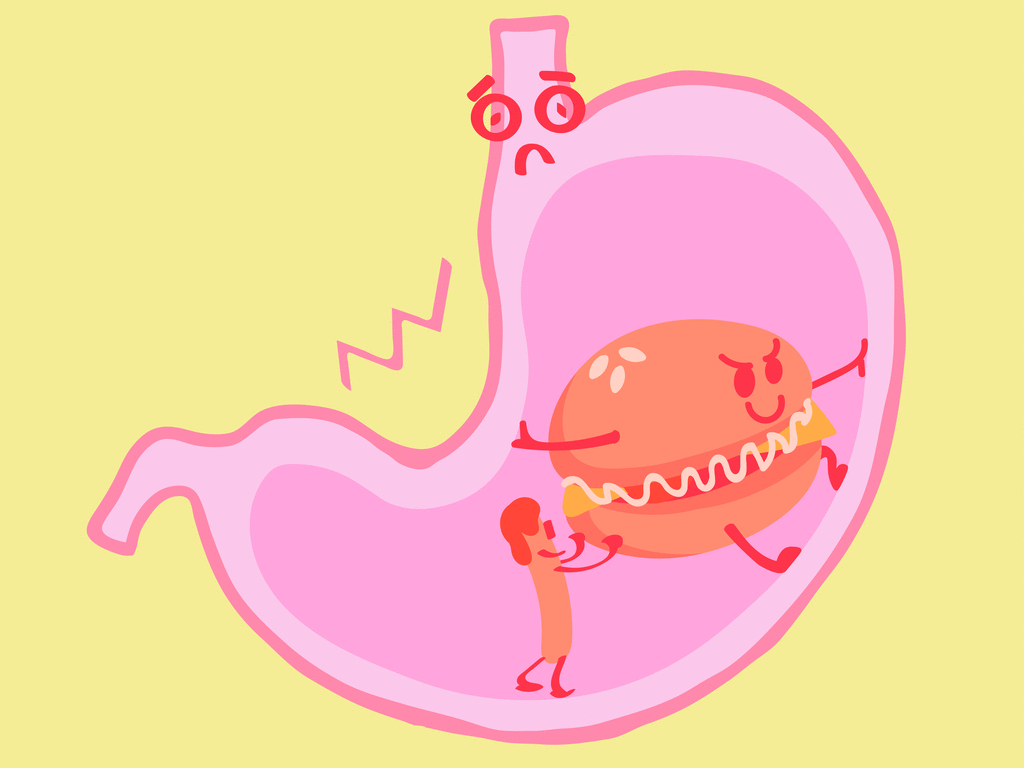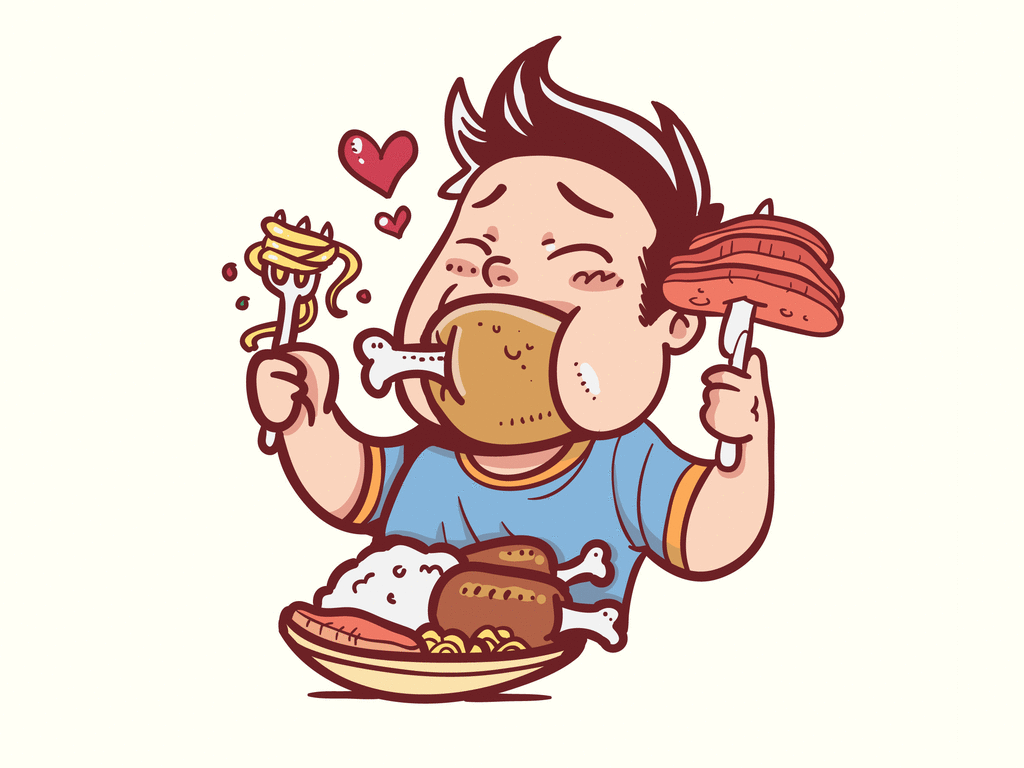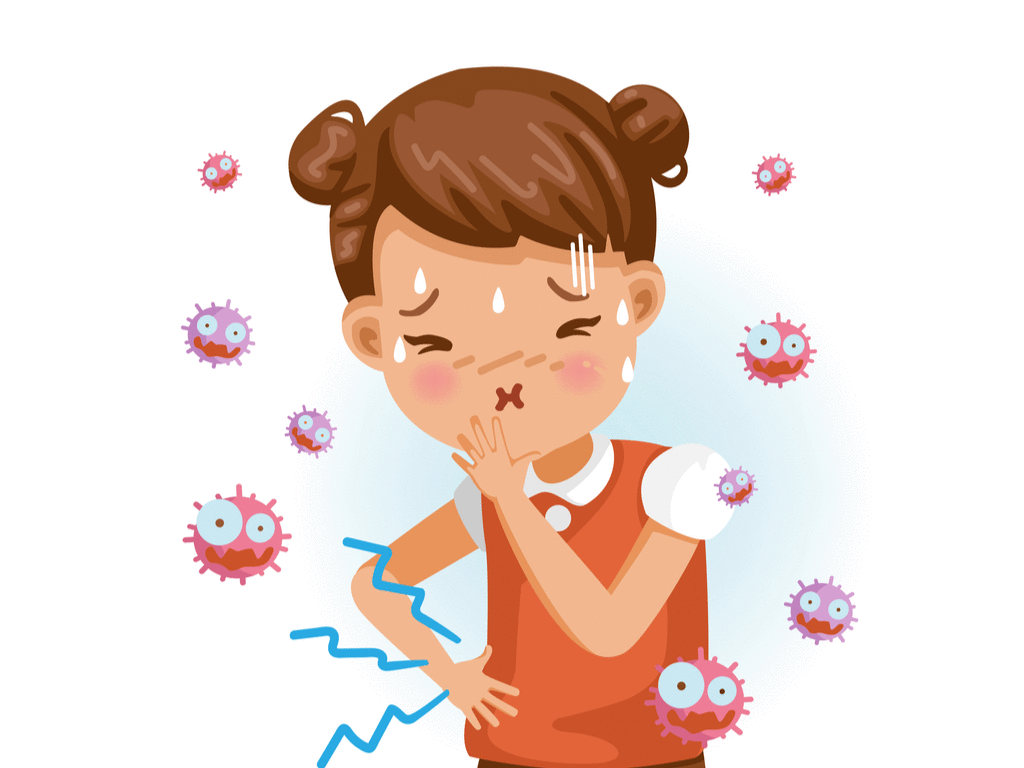
In order to reduce the acidity content, we can drink water which will be diluted; in some cases, the need for antacid is a must which will neutralise the strong acid; we can take antacid such as milk of magnesia, which contains magnesium hydroxide.
2. Ant's bite:
Siddharth and Vishnu went to trucking in the mountains, and accidentally both of them was bitten by a colony of ants. Their travel guide applied baking soda to the wound.
Do you know why?

Applying the baking soda neutralises and reduce the effect of the ant's sting. Because ant's sting contains formic acid, baking soda (sodium hydrogen carbonate) is a basic substance capable of neutralising the wound.
3. Factory wastages:

The factory wastes are neutralised by adding basic substances. Because most factory wastages contain high acidic content, if it is released into the ecosystem, it will affect the organisms which live in that ecosystem and alters it.
4. Tooth decay:
We daily use toothpaste to clean our teeth. Have you ever wondered why?
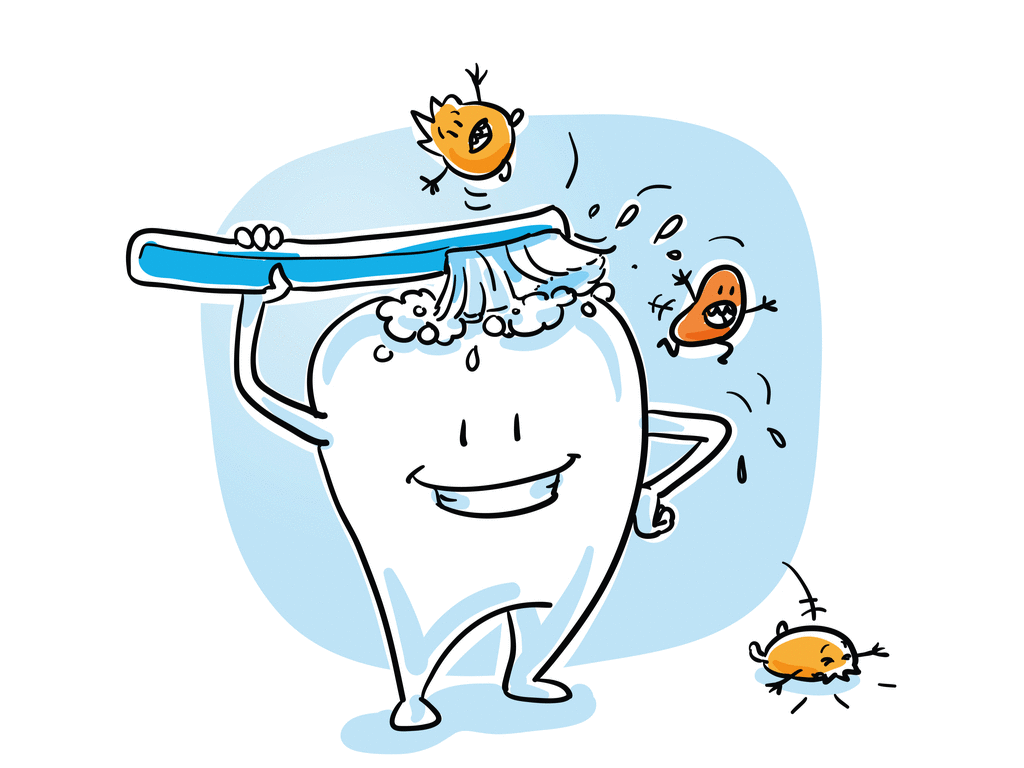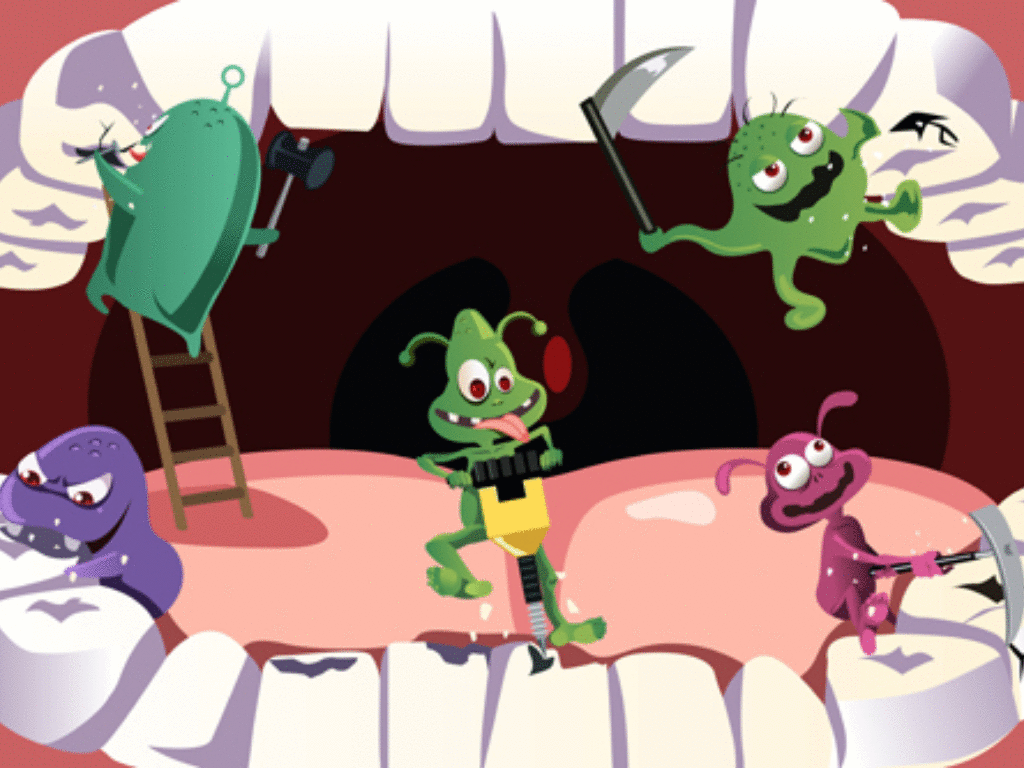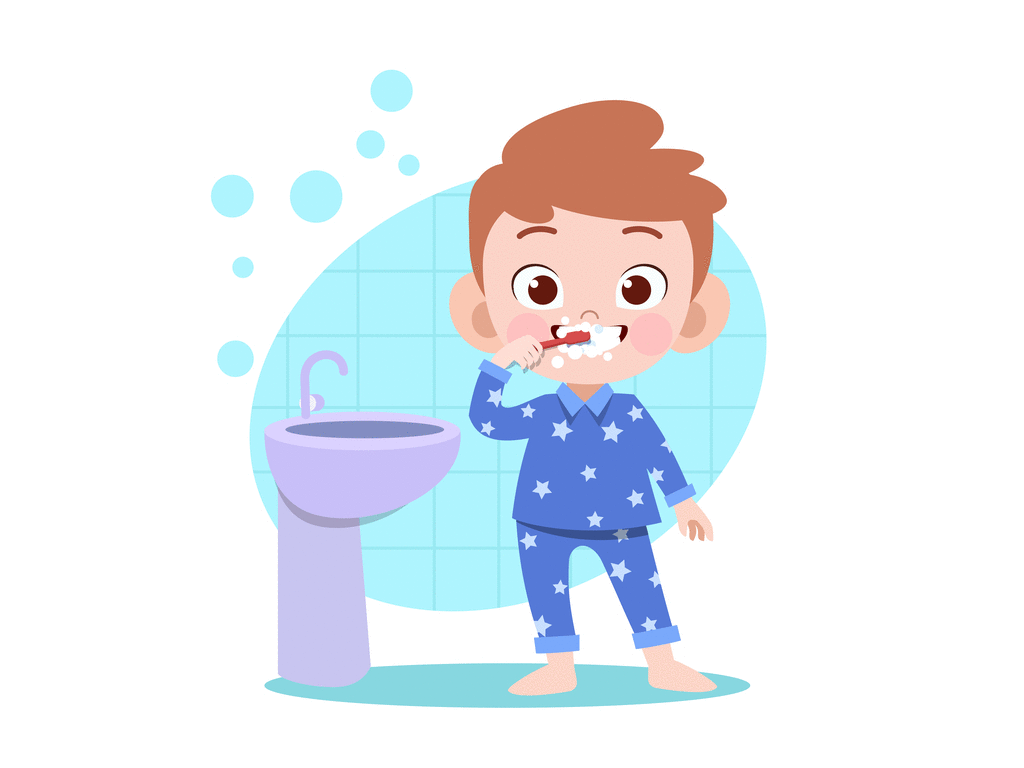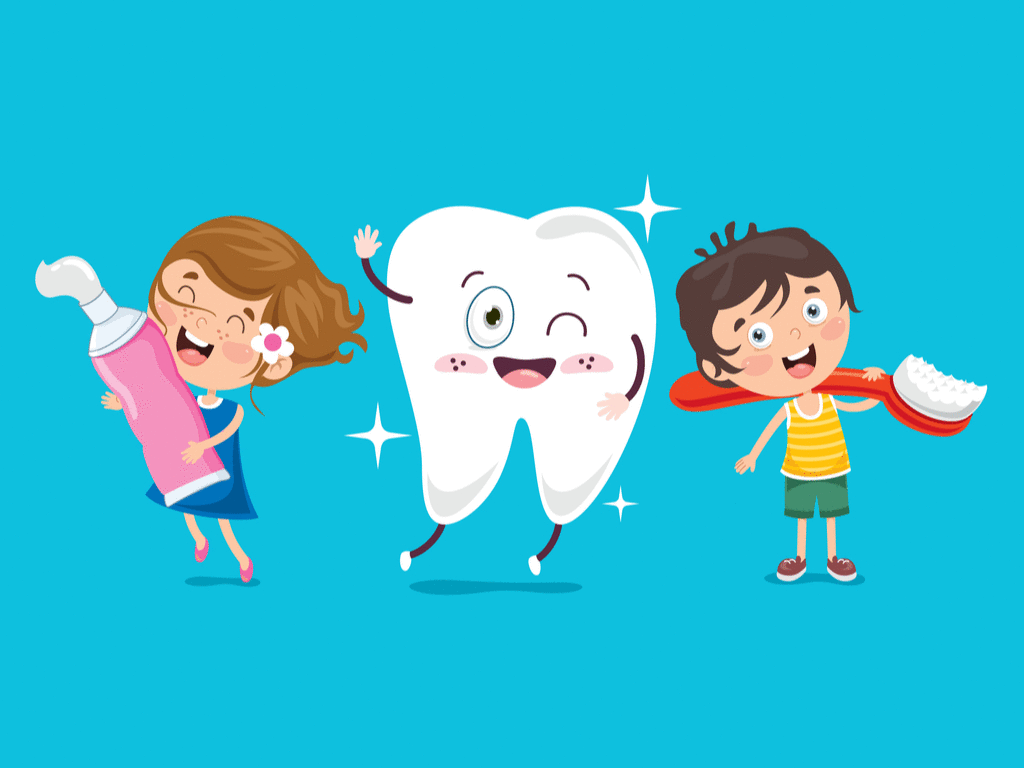
The food we eat will decompose by the microorganism in our mouth, which leads to the formation of acidic content in our mouth. The toothpaste we use contains the basic substance that is capable of neutralising the acidic concentration in our mouth. Most of the substances we use are basic, which do not harm the skin.
5. Soil treatment:

The use of chemical fertilisers in excess causes the soil to become acidic. Plants cannot grow in soil that is either too acidic or too basic. The soil is treated with bases like fast lime (calcium oxide) or slaked lime when it becomes too acidic (calcium hydroxide).
Organic matter (compost) is added to the soil if it is too essential. Organic matter produces acids that help to restore the soil's basic nature.