
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe have seen what acids, bases and neutral solution are. Have you thought about how come these are classified?
We know the taste of acidic and basic substances. Can we taste all of these?
No, It's because acids are corrosive and also some of the strong bases. To confirm a solution to be acid, base or neutral, we use a pH scale to identify them.
No, It's because acids are corrosive and also some of the strong bases. To confirm a solution to be acid, base or neutral, we use a pH scale to identify them.
Acids: The substances that contain more of ions are acidic substances or acids. These are corrosive in nature.

Bases: The substances that contain more of ions are basic substances or bases. These are not corrosive in nature.
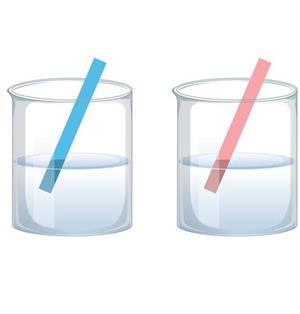
Neutral solutions: These are those that do not change the appearance of either the red or blue litmus paper during the test. These compounds aren't acidic or basic in any way.
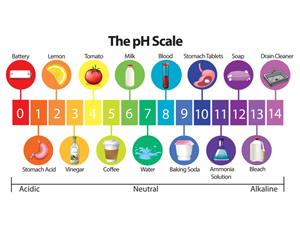
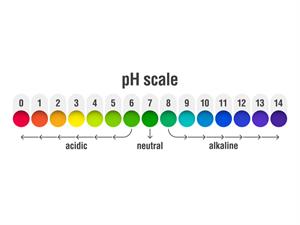
The abbreviation for pH refers to the potential of hydrogen or the power of hydrogen. The pH scale ranges from \(0\) to \(14\), with \(0\) being the most acidic and \(14\) being the most alkaline. The pH of a solution determines whether it is acidic or alkaline.
Litmus paper is paper that has been treated with a natural water-soluble dye so that it can be used as a pH indicator.
Acids contain hydrogen ions and thus lower pH, whereas bases contain hydroxide ions and therefore increase pH.
This pH test determines how many hydrogen ions are present in a given solution. A low pH (acidic substances) is produced by high hydrogen ion concentrations, while a high pH is produced by low hydrogen ion concentrations (basic substances).
Reference:
https://www.shutterstock.com/image-vector/litmus-paper-test-results-possible-color-1920985472