PDF chapter test TRY NOW
Conduction:
In conduction heat transfer takes place between two ends of the same solid or between two solid substances that are at different temperatures but in contact with each other. Thus, we can define conduction as the process of heat transfer that takes place in solids from the higher temperature region to the lower temperature region without any actual movement of atoms or molecules.
Particles with higher kinetic energy transfers thermal energy towards particles with the lower kinetic energy. High-speed particles clash with particles moving at a slow speed; as a result, slow speed particles increase their kinetic energy. This is a typical form of heat transfer that takes place through physical contact.
Heat conduction is a process in which heat is transferred from the hotter part to the colder part of a body without involving any actual movement of the molecules of the body.
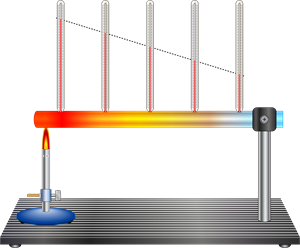
Fig. - Example for conduction
Example:
The following are some of the examples of conduction in daily life.
- In our home, we cook food in vessels that are made up of metals. When the vessel is heated, heat is transferred from the metal to the food.
- When we iron dresses, heat is transferred from the iron box to the cloth.
Based on the substance's ability to conduct the heat, substances are classified as,
- Conductors and
- Insulators
Conductors:
Substances that conduct heat easily are called as conductors. All metals are good conductors of heat.
Insulators:
Substances that do not conduct heat are called as insulators. Wood, cork, cotton, wool, glass, rubber, etc., are insulators.
Example:

Fig. - Example for conductors and insulators
- Cooking vessels are made up of metals because they are good conductors of heat.
- Handles of cooking vessels are made up of plastic or wood because they are poor conductors of heat.
