
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe flow of heat is the process in which energy (thermal energy) is transferred from one object to another.
Whenever two systems come into contact, one system's energy is transferred to another to attain equilibrium. In this case, heat or thermal energy is transferred. Generally, the direction of flow is determined by the temperature. Here, the heat is transferred from the hotter object to the colder object.
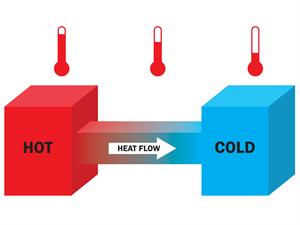
Fig. - Heat flow
What happens when heat is transferred from a hotter object to a colder object?
When two objects come into thermal contact, the hotter object's heat energy is transferred to the colder object inducing an increase in kinetic energy. Due to this, increase in kinetic energy, molecules in the colder object start to move faster, resulting in an increase in temperature. This process continues until thermal equilibrium is achieved.
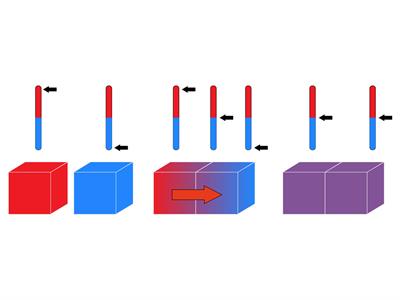
Fig. - Flow of heat from hotter object to colder object
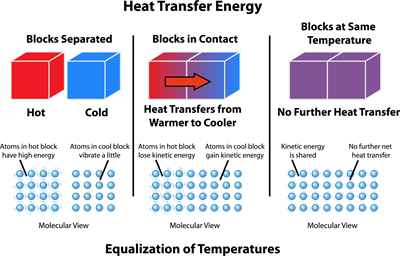
Fig. - Heat transfer energy
The above diagram shows how the heat transfer takes place between two objects. The diagram is divided into three columns.
- First, one shows two objects with different temperatures and are not in contact.
- The second column shows that the two objects are in thermal contact, and heat transfers from the hot object to the cold object. It also indicates that the atoms in the hot body lose their kinetic energy which is being transferred to the cold body.
- The third column shows that thermal equilibrium is achieved, and there is no transfer of heat hereafter.
Thermal Contact:
Two systems are said to be in thermal contact if they can exchange energy through the process of heat transfer.
Thermal Equilibrium:
Two systems are said to be in thermal equilibrium if there is no heat transfer between them, and both have the same temperature.