
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWhen an electric current is passed through the conducting solution, a chemical reaction occurs. The chemical reaction that occurs depends upon the solution is taken and the metal used in the electrodes. As a result,
- bubbles of gas may form
- the colour of the solution may change
- deposition of the metal occurs at the electrode
These are the chemical effects of electricity.
Formation of bubbles of gas due to electric current:
- Take two carbon rods length of \(6\) cm (carbon rod we get from inside a pencil). These rods are called electrodes. Electrodes are substances that conduct electricity and connect the non-metallic part of the circuit.
- Connect the terminals of the battery to the rods using a wire.
- Pour water into a plastic or glass container.
- Add some salt or lemon juice to the water for more conduction (solution is more conducting than ordinary water).
- Electrodes to be placed into the solution.
- When the reaction takes place, small bubbles are evolved at the end of the electrodes.
- These bubbles are called oxygen.
This is one of the chemical effects of electricity.
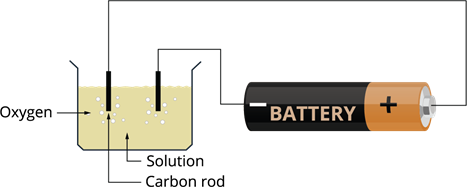
Experiment setup
The above process is called electrolysis.
In \(1800\), William Nicholson, a British chemist, found that when electrodes were immersed in water and electricity was passed, bubbles of oxygen and hydrogens were evolved. Oxygen was deposited at the electrode connected to the positive terminal of the battery, and hydrogen was deposited at the electrode connected to the negative terminal of the battery.
Colour change due to the electric current:
- Take a potato and cut it into two halves.
- Insert the copper wires of the tester into it.
- After an hour, we can see a bluish-green spot on the potato.
- This spot occurs around the copper wire connected to the positive terminal of the battery, whereas no such spot occurs at the other wire.
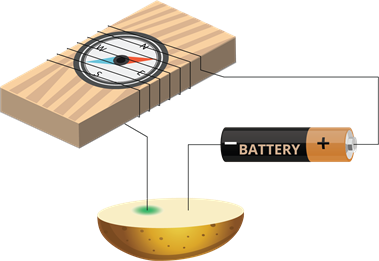
Experiment setup
Application:
This experiment is used to identify the positive terminal of a cell or battery in a concealed box.