PDF chapter test TRY NOW
In general, the material which conducts electricity is called a conductor.
The materials which allow an electric current drive through them are called good conductors.
Example: Metals, such as copper and aluminium.
The materials which do not permit the electric current drive through them are called poor conductors or insulators. Thus, the insulators are used to protect us from the electric current.
Example: Non-metals, such as plastic, rubber and wood.
Example: Metals, such as copper and aluminium.
The materials which do not permit the electric current drive through them are called poor conductors or insulators. Thus, the insulators are used to protect us from the electric current.
Example: Non-metals, such as plastic, rubber and wood.
The materials that have a conductivity between conductors and insulators are called semiconductors.
Example: Ceramics, silicon, germanium, gallium arsenide and cadmium selenide.
Example: Ceramics, silicon, germanium, gallium arsenide and cadmium selenide.
How to check whether a material conducts an electricity?
A tester is equipment, which is used to check whether a material conducts electricity or not.

Before testing a material, we need to ensure that,
- whether the tester is working
- the connections should not be loose
- cells are not used up
- the bulb is not fused
- the solution taken is a good conductor
In Class \(VI\), we have tested the materials in the solid-state using a tester. Let us see the conductivity of liquids.
The conductivity of liquid:
All liquids are not conductors of electricity. The liquids which allow electricity to pass through them are called electrolytes.
We can produce electricity using electrolytes. The electrolytes are either compound or molten salt.
Most of the materials can conduct electricity under certain conditions. In this way, it is classified into good and poor conductors instead of conductors and insulators.
Most of the materials can conduct electricity under certain conditions. In this way, it is classified into good and poor conductors instead of conductors and insulators.
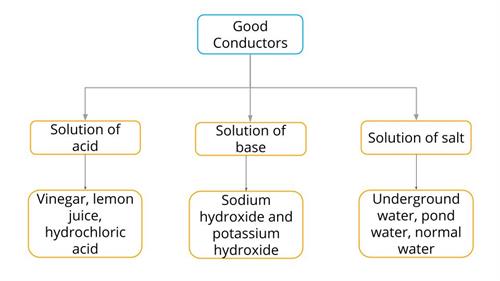
Examples of good conductors
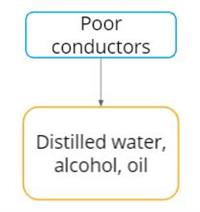
Poor conductors
