
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWhen our mother makes gravy, we can see that she adds salt and spice in a certain ratio. It is only when we add salt and spice and other ingredients at a certain ratio we get a scrumptious gravy.
We can apply this similar situation to the chemical compounds. The great scientist Lavoisier, along with other scientists, observed that compounds are composed of two or more elements; and each such compound had the same elements in the same proportions, irrespective of where the compound came from or who prepared it.
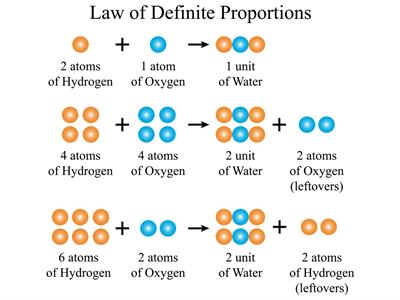
Example:
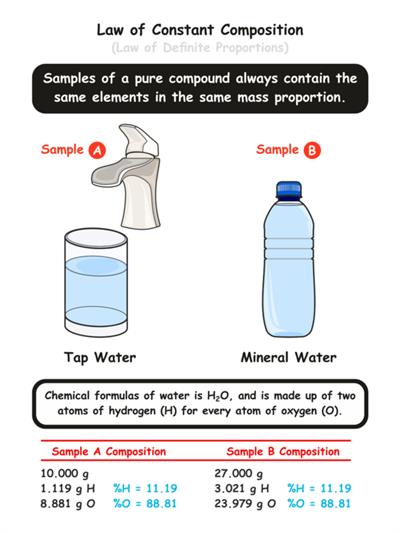
For example, In a compound such as water, the ratio of the mass of hydrogen to the mass of oxygen is always \(1:8\), irrespective of the classification of water (groundwater, bottled water, rainwater and so on).
So, if \(9 g\) of water is decomposed, \(1 g\) of hydrogen and \(8 g\) of oxygen is always obtained. If \(18 g\) of water is decomposed, we can obtain \(2 g\) of hydrogen and \(16 g\) of oxygen.
Similarly, in \(HCl\) compound is composed of one to one ratio of hydrogen and chlorine.
All these led to the law of constant proportions, which is also known as the law of definite proportions.
According to the law of constant proportions stated by Proust,
“The elements are always present in definite proportions by mass in a chemical substance.”
Law of constant proportions
The arrival of John Dalton:

John Dalton
Scientists faced a tough situation to give appropriate explanations of these laws.
In the upcoming lesson, we will see John Dalton’s postulates.
But then, John Dalton came up with an idea of the divisibility of matter. His theory was based on the laws of chemical combination. Dalton’s atomic theory explained the law of conservation of mass and the law of definite proportions.