
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIn everyday life, the products we use, such as mayonnaise, jelly, and butter are the colloidal solution.

Colloidal solution examples
Colloidal Solution:
A colloidal solution is a heterogeneous mixture. In the colloidal solution, the particles of a colloid uniformly spread throughout the solution.
A colloidal solution contains finely divided particles (Approximately \(1\) to \(1000\) millimicrons in size), dispersed within a continuous medium in such a manner that prevents them from being filtered easily or settled rapidly.
A colloidal solution contains finely divided particles (Approximately \(1\) to \(1000\) millimicrons in size), dispersed within a continuous medium in such a manner that prevents them from being filtered easily or settled rapidly.
Example:
1. Milk
2. Hair cream
3. Toothpaste
4. Fog, Mist, Smog
5. Cheese butter
6. Paint etc.
Properties of a Colloidal Solution:

1. Colloidal solution is a heterogeneous mixture.
2. A colloidal solution is quite stable so that it does not settle down when left undisturbed.
3. Though the size of the particle here is so tiny, you cannot see with naked eyes.
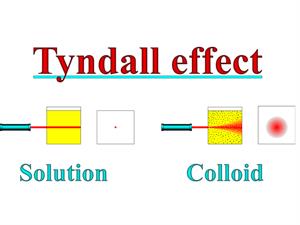
4. Colloidal particles can scatter a beam of light passing through it and make its path visible.
Types of Colloidal Solutions:
Classification of colloids based on the physical state of the dispersed phase and dispersion medium.
(a). Dispersed phase: The dispersed phase is described as a phase that is scattered or present in the form of colloidal particles.
(b). Dispersed medium: The dispersed phase is the medium in which colloidal particles are distributed.

S.No | Name | Dispersed phase | Dispersed medium | Example |
1. | Solid sol | Solid | Solid | Colored glass, Gems, Alloys. |
2. | Sol | Solid | Liquid | Paint, Fruit jellies, Dye, Ink, Egg white |
3. | Aerosol | Solid | Gas | Smoke |
4. | Gel | Liquid | Solid | Cheese, Butter. |
5. | Emulsion | Liquid | Liquid | Milk, Oil in water, Mayonnaise, Face cream. |
6. | Aerosol | Liquid | Gas | Fog, Mist, Clouds, Body sprays. |
7. | Foam | Gas | Liquid | Soap lather, Shaving cream, Coffee froth |
8. | Solid foam | Gas | Solid | Rubber, Sponge, Cake, Bread |