PDF chapter test TRY NOW
Mixture:
A mixture is made up of one or more elements or compounds blended together rather than chemically combined.
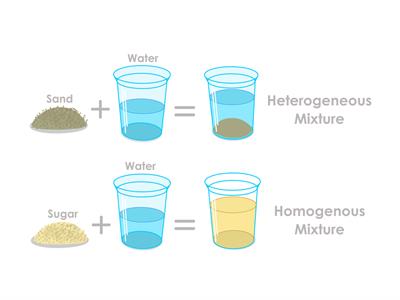
Types of Mixtures
- A mixture's constituents do not exist in a predetermined ratio.
- It is subject to change.
- In nature, mixtures can be either homogeneous or heterogeneous.
- Physical methods are used to distinguish mixture constituents.
- The constituents in a mixture retain their identities, i.e., a mixture displays all of the constituents' characteristics.
- Smog = smoke + fog. Therefore, it is an example of the mixture.
Compounds:
It is a form of matter created by combining two or more elements in a specific mass ratio. To decompose it into its constituent components, we use chemical methods.
Compound formation
- The elements in a compound are present in a fixed mass ratio, and this proportion will not change.
- Compounds are often homogeneous, meaning that their composition is consistent throughout.
- Physical methods cannot isolate the constituents of a compound.
- The constituents of a compound lose their identities, i.e., the compound loses its identity.
- Water \(H_2O\) is an example of a compound; as we chemically separate these elements, their properties differ from water.
Example:
Lets now see the difference between compounds and mixtures with the help of an activity:
Step 1: Combine powdered iron filings and sulphur in a mixing bowl.
Step 2: Make two equal portions using the mixture.
Step 3: The first half of the mixture should be kept separately, while the second half should be heated.
Iron + sulphur \(\overset{Heating}{\rightarrow}\) Iron sulphide
Observation: When heated, a black brittle substance (iron sulphide) forms.
Result: The iron sulphide (compound) that forms has completely different characteristics than the iron and sulphur combination.
Iron sulphide \(=\) compound
Here when the magnetic field is applied, it has no effect.
Iron + sulphur \(=\) mixture
Here when the magnetic field is applied, the iron powder gets attracted.
Thus, from the above activity, we proved that the qualities of the elements of a mixture are displayed whereas the qualities of the individual elements are not visible in compounds.
There are many complex mixtures under homogeneous and heterogeneous type, such as solutions, alloys, suspensions, and colloids, which will be seen in the upcoming sessions.
