PDF chapter test TRY NOW
There are certain solid substances like camphor, naphthalene, menthol, dry ice (solid form of carbon dioxide) that get converted into gas directly upon heating without becoming liquid.

Naphthalene/Mothballs sublimation
This change in the state during the sublimation process is due to the change in temperature of the substances.

Burning camphor
Sublimation:
Sublimation occurs when heated, and hence it is called an endothermic process. The process where solid directly changes into vapour on heating is known as sublimation.
Sublimation occurs in a substance when the vapour pressure (gas pressure exerted by vapours of the substance) of that substance is high. This method is used for the purification of solids.
This is a physical change as no new substance is formed, and the vapours formed, when collected and cooled, the substance will be back to its original state without any change in their chemical properties.
Example:
Let us see how we can separate a salt mix with camphor? Step 1: The impure substance is placed in a watch glass and covered with an inverted funnel. This setup is placed on the tripod stand.
Step 2: The end of the inverted funnel is covered with cotton.
Step 3: Then, the China dish is heated.
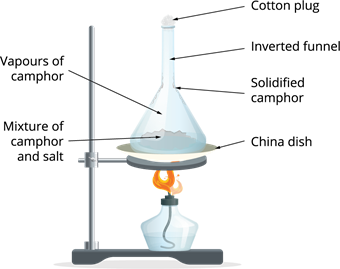
Sublimation process
Observation: When heated, camphor transforms from a solid to a gaseous state leaving behind the salt.
Result: The sublimation method is used to isolate certain mixtures that contain a sublimable volatile component from a non-sublimable impurity.
Ammonium chloride, naphthalene, and anthracene are few examples of solids that sublimes.
