PDF chapter test TRY NOW
Write brief answers to the following questions:
1. A sample of water under study was found to boil at 102°C at normal temperature and pressure. Is the water pure? Will this water freeze at 0°C? Comment.
The sample of water boils at a which shows that water is not pure. Due to impurities present in it water boils at a higher temperature. This water will freeze below .
2. A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figure would correctly represent the result? Justify your choice.
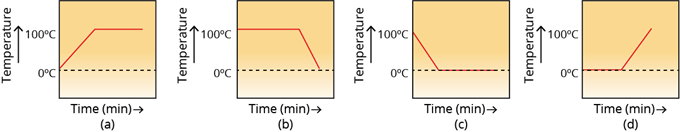
Since ice and water are in equilibrium, the temperature would be . When we heat the mixture, energy supplied is utilized to melt the ice, and the temperature does not change until all the ice melts because of the latent heat of fusion. On further heating, the temperature of the water would increase. Therefore the correct option is .
