
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIsobars:
Isobars are atoms with different atomic numbers but the same mass number. In other words, the nucleon count is the same, but the number of protons is different.
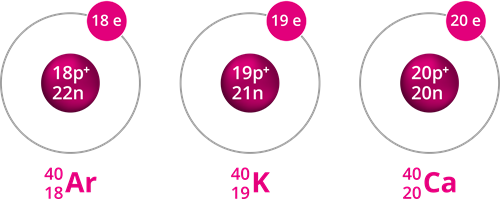
Example for Isobars
Difference between Isotopes and Isobars:
|
Isotope
|
Isobar
|
| Same atomic numbers but a different mass number. | Different atomic numbers but the same mass number. |
|
The chemical properties are identical.
|
The chemical properties are different. |
| Physical properties differ from one another. | The physical properties are the same. |
| The number of protons and electrons are the same, but the number of neutrons differs. | The number of protons, electrons and neutrons differs. |
| Examples: | Examples: |
Isotones:
Isotones are atoms of different elements with different atomic numbers and mass numbers but the same number of neutrons.
Example: and
Number of neutrons in boron \(=\) \(11\) \(-\) \(5\) \(=\) \(6\)
Number of neutrons in carbon \(=\) \(12\) \(-\) \(6\) \(=\) \(6\)
The above pair of elements, boron and carbon, has the same number of neutrons but different numbers of protons, resulting in different atomic numbers.
Other examples:
1.
| (p \(=\) \(6\), n \(=\) \(8\)) | |
| (p \(=\) \(8\), n \(=\) \(8\)) | |
| (p \(=\) \(7\), n \(=\) \(8\)) |
2.
| (p \(=\) \(9\), n \(=\) \(10\)) | |
| (p \(=\) \(10\), n \(=\) \(10\)) |
3.
| (p \(=\) \(11\), n \(=\) \(12\)) | |
| (p \(=\) \(12\), n \(=\) \(12\)) |
Points to remember:
- The structure of an atom has been proposed by various scientists, as shown below:
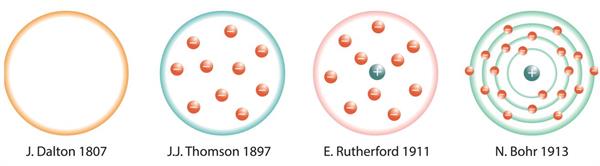
Atom Models
- The sub-atomic particles of an atom are:
Subatomic particles
- The shells or energy levels of an atom are represented by K, L, M, N, etc.
- The combining capacity of an atom is called valency.
- Atomic number = Number of protons in the nucleus
- Nucleons are protons and neutrons in the nucleus.
- Mass number = Number of nucleons
- Isotopes are atoms with the same atomic number but different mass numbers.
- Isobars are atoms of the same mass number but different atomic numbers.