PDF chapter test TRY NOW
We know that,
- Atoms have protons and neutrons in their nuclei.
- Neutrons have no charge, whereas protons have a positive charge.
- The magnitudes of positive and negative charges in an atom are equal, resulting in an electrically neutral atom.
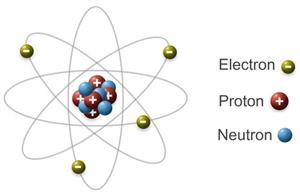
Structure of an atom
Atomic Number:
The number of protons in the nucleus of an atom is known as its atomic number.
The symbol "Z" stands for the atomic number. The atom of a different element has a different number of protons.
The symbol "Z" stands for the atomic number. The atom of a different element has a different number of protons.
The number of electrons and the number of protons is equal in a neutral atom.
Example:
The atomic number of carbon is \(6\); hence, the number of protons in the nucleus is \(6\). Similarly, the atomic number of nitrogen is \(7\); hence, the number of protons in the nucleus is \(7\).
We can easily calculate the number of electrons or protons in an atom if we know its atomic number. Let us recall the points for that.
- The number of protons is known as the atomic number.
- In a neutral atom, the number of protons is equal to the number of electrons.
Example: For \(O\), calculate the number of (i). protons and (ii). electrons.
Atomic number of oxygen = 8.
(i). Protons
We know, the atomic number is equal to the number of protons.
Hence, the number of protons = \(8\)
(ii). Electrons
In a neutral atom, the number of protons are equal to the number of electrons.
Hence, the number of electrons = \(8\)
Mass Number:
We have studied that the mass of an atom is concentrated in its nucleus because the size of the electron is negligible.
A mass number or atomic mass of an atom is equal to the sum of the number of protons and neutrons present in the nucleus. Hence, the protons and neutrons are also called Nucleons.
(or)
The mass number or atomic mass is represented by the symbol "A".
By rearranging the atomic mass formula, we can calculate the number of protons, neutrons, or atomic number.
(or)
(or)
Example: Carbon
Number of protons = \(6\)
Number of neutrons = \(6\)
Hence, the mass number = \(6\) + \(6\) = \(12\ u\)
The atomic number (Z), mass number (A) and symbol of an element are written as follows in atomic notation:
Where, X is the symbol of element.
A = Protons + Neutrons
Z = Protons or electrons
